A pregnant woman’s Rh factor (an immunoglobulin protein) is important information to know. If a woman’s blood is Rh negative and her baby’s is Rh positive, this could cause problems for her future pregnancies if left untreated. As OBGYN Dr. Ingrid Skop explained (emphasis added), “Evaluation of Rh status and provision of Rhogam, if indicated, has long been the standard of care for early pregnancy loss, including elective induced abortion. This action will prevent a mother from mounting an immune response to her future unborn children. If Rhogam is not given and isoimmunization occurs, 14% of untreated infants will be stillborn and half will suffer neonatal death or brain injury.”
This is why new Rh testing and treatment updates published by The American College of Obstetricians and Gynecologists (ACOG) — a group tied to Big Abortion — are raising eyebrows. ACOG’s new guidelines no longer recommend testing or treatment for Rh-negative blood in women who abort at 12 weeks of pregnancy or less.
The reason?
Because there are “concerns that provision [of testing and treatment] can delay or impede access to abortion care.”
Perhaps not so coincidentally, the changes come at a time when abortion businesses are also administering no-test abortion pills to women up to… you guessed it… 12 weeks — two weeks later than what the FDA currently approves.
The timing is suspect
Live Action News has previously documented how the abortion industry used the COVID-19 pandemic to lift the in-person safety regulations under the REMS safety regulations on the abortion pill mifepristone, expanding access to the abortion pill. In fact, well before the pandemic, the abortion industry expanded its abortion pill clinical trials and then rolled out a “no test” abortion pill protocol — eliminating important testing, bloodwork, and ultrasounds, which some medical professionals assert endangers women.
Around the same time frame, the ACOG also changed its recommendations to coincide exactly with the abortion industry’s attempts to expand access to the abortion pill.
ACOG’s update replaces the organization’s previously published Practice Bulletin No. 225, (Medication Abortion Up to 70 Days of Gestation), Practice Bulletin No. 200 (Early Pregnancy Loss), and Practice Bulletin No. 181 (Prevention of Rh D Alloimmunization.) The updated Clinical Practice Update, “Rh D Immune Globulin Administration After Abortion or Pregnancy Loss at Less Than 12 Weeks of Gestation,” was published September 10, 2024, in the organization’s Green Journal. The update claims to “provide[] revised guidance on Rh testing and Rh D immune globulin administration for individuals undergoing abortion or experiencing pregnancy loss at less than 12 0/7 weeks of gestation.”
ACOG’s claim that “there is a very low likelihood of Rh alloimmunization associated with abortion or pregnancy loss at less than 12 weeks of gestation” is suspect, given that abortion facilities and Planned Parenthood centers are now openly offering the abortion pill regimen for DIY self-managed abortions at home, through 12 weeks of pregnancy — past the Food and Drug Administration’s (FDA) approved 10-week gestational limits.
Additionally, ACOG’s changes follow an announcement of shortages of RhoGAM, the prescription medicine used to prevent Rh immunization. Ironically, prescribing information on the RhoGAM website contradicts ACOG’s change and suggests that RhoGAM or MICRhoGAM should be prescribed for “the [a]ctual or threatened termination of pregnancy (spontaneous or induced) up to and including 12 weeks gestation” and should be “[a]dminister[ed] within 72 hours.”
“Clinical studies demonstrated that administration of MICRhoGAM within three hours following pregnancy termination was 100% effective in preventing Rh immunization,” the prescribing document added.
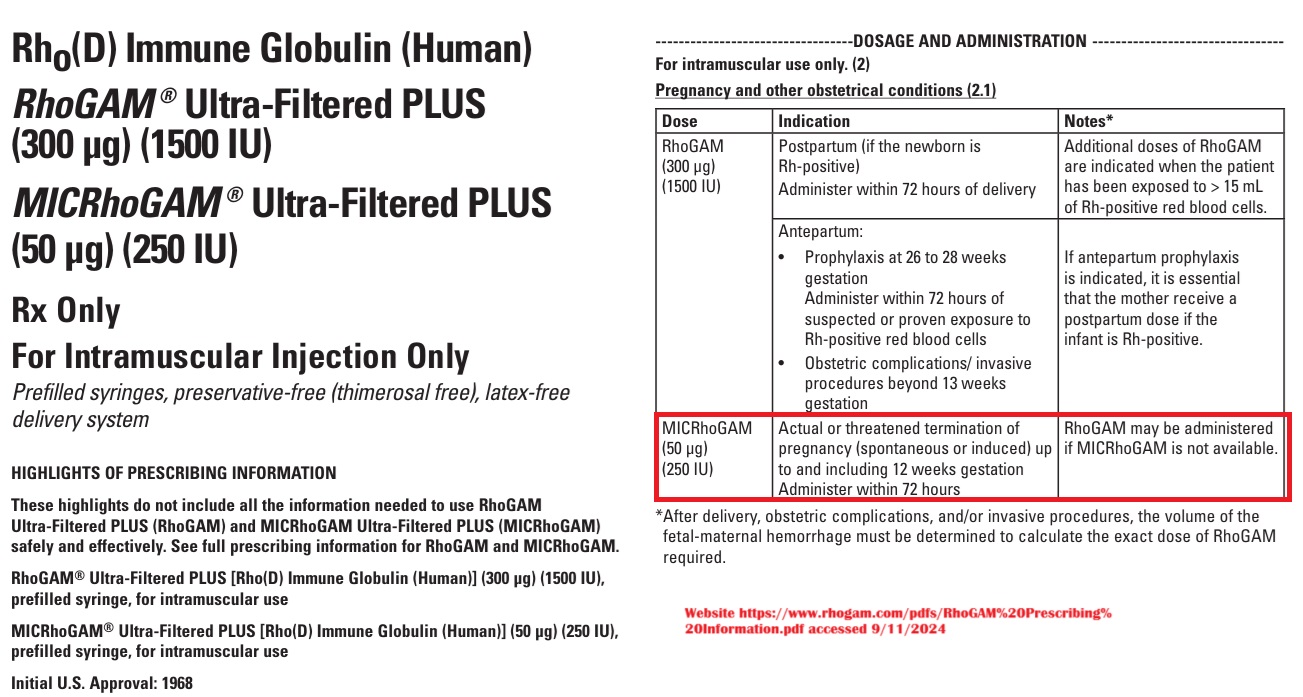
RH Negative RhoGAM drug prescribing document
Previous requirements
ACOG’s previous Practice Bulletin (225) stated in part, “Before medication abortion is performed, the clinician should confirm pregnancy and estimate gestational age. For patients with regular menstrual cycles, a certain last menstrual period within the prior 56 days, and no signs, symptoms, or risk factors for ectopic pregnancy, a clinical examination or ultrasound examination is not necessary before medication abortion.”
“Rh testing is recommended in patients with unknown Rh status before medication abortion, and Rh D immunoglobulin should be administered if indicated. In situations where Rh testing and Rh D immunoglobulin administration are not available or would significantly delay medication abortion, shared decision making is recommended so that patients can make an informed choice about their care. Other laboratory evaluations are not routinely indicated but may be required by local and state laws…” the Bulletin read.
A March 2020 document published by Planned Parenthood claims, “Rhesus (Rh) factor is a protein some people have in their blood. If your blood has the protein, you are Rh positive. If your blood does not have the protein, you are Rh negative.”
“During pregnancy, blood cells from the fetus can enter your blood. If you are Rh negative and the fetus is Rh positive, your body can develop antibodies (another kind of protein) against Rh-positive blood… This does not harm you, but it can cause serious problems if you get pregnant again. Rh antibodies can attack and destroy the blood of a Rh-positive fetus. It can give the fetus a very bad anemia. It may also lead to many other serious problems,” Planned Parenthood added.
Planned Parenthood’s glossary claimed the condition could be “dangerous.”
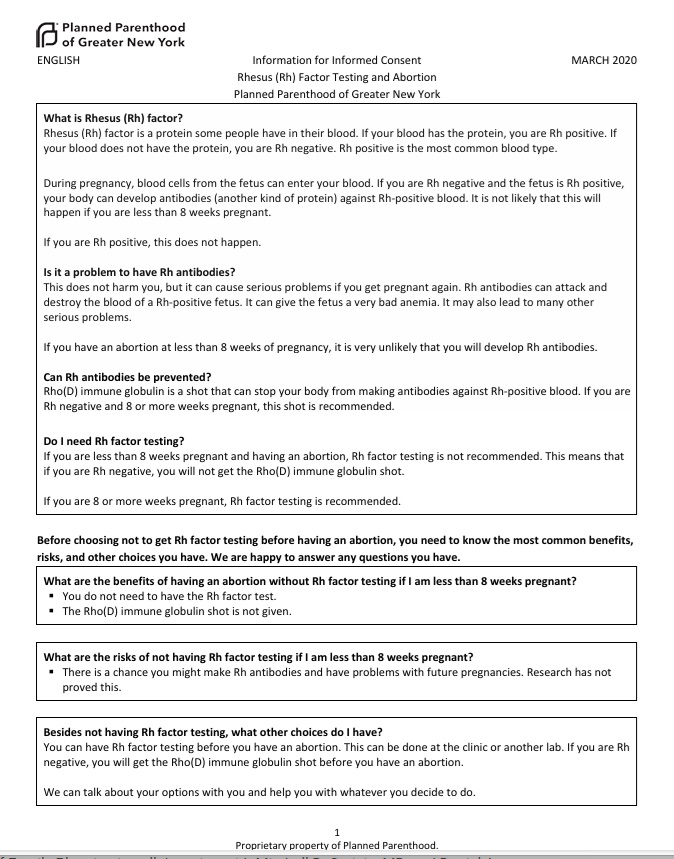
Planned Parenthood on RH negative blood March 2020
In addition, a handout previously provided by the pro-abortion group Advancing New Standards in Reproductive Health (ANSIRH), produced for an online for pharmacy training course in medication abortion, stated “consider testing if >8 weeks gestation and patient is interested in RhD immunoglobulin, if needed.”
The training video, “Pharmacists’ Roles in Medication Abortion,” contains the screenshot below. In the video, Dr. Daniel Grossman tells pharmacists that “Patients who are Rh negative who may be pregnant with an Rh positive embryo or fetus need to consider the potential risks…”
“After eight weeks if the patients does not know their Rh status, a blood test may be ordered, and if the patient is Rh negative, they may be given RhD immunoglobulin also known as RhoGAM,” says Grossman, who is an abortionist pushing for expansion of the abortion pill.
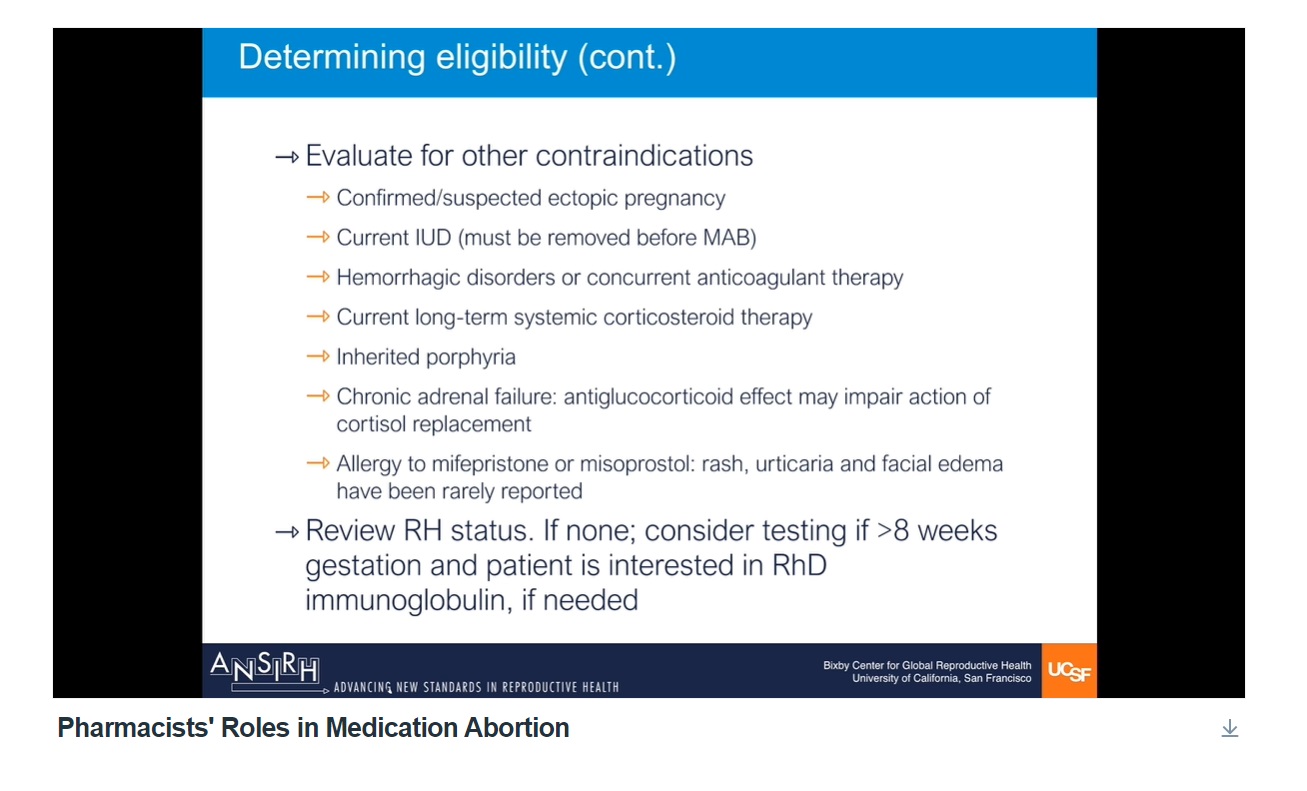
RhNegative ANSIRH screen in pharmacy class provided by Grossman
According to VerywellHealth.com, “The risk to Rh positive fetuses and babies carried by an Rh negative pregnant person is higher for those carried after the first pregnancy.”
Separately, ACOG states: “Within 72 hours after the delivery of an Rh-positive baby — The greatest chance that the blood of an Rh-positive fetus will enter the bloodstream of an Rh-negative woman happens during delivery. RhIg prevents an Rh-negative woman from making antibodies that could affect a future pregnancy. The treatment is good only for the pregnancy for which it is given. Each pregnancy and delivery of an Rh-positive baby requires a repeat dose of RhIg.”
New requirements
ACOG claimed to base its decision “mainly on expert opinion and indirect evidence of RhIg [Rh D immune globulin] benefit in full-term pregnancies” which ACOG “and many other medical organizations historically recommended routine administration of RhIg to all Rh-negative unsensitized patients after abortion or pregnancy loss at less than 12 weeks of gestation.”
ACOG claimed “This was because the potential benefit of preventing Rh alloimmunization, which, if left untreated, can lead to significant perinatal morbidity and mortality in future pregnancies, was thought to outweigh the negligible medical risks and additional economic costs associated with Rh testing and RhIg administration.”
However, ACOG now claims that “findings from recent studies suggest that there is a very low risk of Rh alloimmunization associated with abortion or pregnancy loss at less than 12 weeks of gestation (8–10), and many expert guidelines no longer recommend routine RhIg prophylaxis for abortion or pregnancy loss at less than 12 weeks of gestation because of the lack of evidence to support benefit and concerns that provision can delay or impede access to abortion care.
(You’ll recall, however, that ACOG admittedly made the changes out of “concerns that provision can delay or impede access to abortion care.”)
The document reiterates:
For patients at less than 12 0/7 weeks of gestation who are undergoing abortion (managed with uterine aspiration or medication) or experiencing pregnancy loss (spontaneous or managed with uterine aspiration or medication): ACOG suggests forgoing routine Rh testing and RhIg prophylaxis.
Although not routinely indicated, Rh testing and RhIg administration can be considered on an individual basis in the context of a shared decision-making discussion about the potential benefits and risks.
Routine Rh testing in patients with unknown Rh status and administration of RhIg continue to be recommended for Rh-negative, unsensitized patients undergoing abortion or experiencing pregnancy loss at or beyond 12 0/7 weeks of gestation (13).
The changes reflect findings in a 2023 JAMA study — paid for by the pro-abortion Society of Family Planning Research Fund. ACOG is also joined by additional pro-abortion organizations, such as the National Abortion Federation’s (NAF) 2024 Clinical Guidelines.

ACOG December 2024 bulletin on RH negative treatment and abortion
Society for Maternal-Fetal Medicine (SMFM) Calls for Caution
Despite ACOG’s move, the Society for Maternal-Fetal Medicine (SMFM) is calling for caution. A statement published at the American Journal of Obstetrics and Gynecology (AJOG), warns that “a perceived choice for or against RhIg administration may persuade insurance companies to view this therapy as optional or without clear benefit when, in fact, there are no high-quality data demonstrating that RhIg is not indicated.”
“Given the substantial impact of alloimmunization on pregnancy and perinatal outcomes, prevention is critical. In addition, the risks associated with RhIg administration are low. Therefore, in care settings in which RhD testing and RhIg administration for unsensitized RhD-negative individuals are logistically and financially feasible, we recommend offering both RhD testing and RhIg administration for spontaneous and induced abortion at <12 weeks of gestation,” SMFM wrote.
Unfortunately, SMFM supports abortion — and despite signaling a legitimate concern, in the end, the organization waffled, placing the immediate killing of the preborn baby through abortion (especially a DIY self-managed abortion) above the safety of future preborn children.
“If requiring RhD testing or RhIg administration could lead to a delay in accessing abortion care or pose financial and logistical barriers to receiving abortion care, then we agree that the priority is to complete the abortion,” SMFM wrote (emphasis added). “Patients should be counseled on the potential implications of an unknown blood type and the possible risks of nonadministration of RhIg. In cases in which RhD testing and RhIg administration are readily available before abortion, we recommend offering testing and administration if indicated. While the available evidence does not conclusively support forgoing RhD testing and RhIg administration for first-trimester abortion, neither does it justify requirements for in-person dispensing of mifepristone or preabortion testing.”
ACOG’s Disclaimer
It is important to understand that ACOG is so beholden to pro-abortion ideology that it appears to prioritize Big Abortion’s agenda to expand abortion, over all else. Live Action News previously documented that:
- ACOG has referred for, advocated for, and promoted abortion for decades.
- ACOG’s goal, from nearly the beginning, has been population control.
- ACOG changes its terminology to more easily promote a pro-abortion agenda to the public.
- Less than 25% of ACOG’s membership in 2019 was willing to provide abortions.
- ACOG is not impartial, and has attempted to “fact check” and even censor those with whom it disagrees.
- ACOG’s goal is to expand abortion, and it is working toward that end.
- ACOG has become part of Big Abortion and is funded by those invested in abortion’s expansion.
ACOG’s Practice update, while granting cover for expanded use of DIY abortion pills, ironically also contains this disclaimer: “While ACOG makes every effort to present accurate and reliable information, this publication is provided ‘as is’ without any warranty of accuracy, reliability, or otherwise, either express or implied. ACOG does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither ACOG nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with this publication or reliance on the information presented.”
While ACOG claims that “All ACOG committee members and authors have submitted a conflict of interest disclosure statement related to this published product,” no author names or conflicts were disclosed in the practice update.







