For several months, pro-abortion groups have been working to expand abortion pill access and to lift the U.S. Food and Drug Administration’s (FDA) safety system known as Risk Evaluation and Mitigation Strategy (REMS). The abortion industry is now pushing a “no test” abortion pill protocol, detailed here and here. While the industry would like the public to believe the “no test” protocol was put in place because of COVID-19, the plan has actually been in the works for a long time.
At a 2019 panel discussion at the taxpayer-funded University of California’s Bixby Center for Global Reproductive Health, abortion providers detailed efforts to remove all testing for women (including ultrasounds, exams, and blood work) before dispensing the abortion pill. At that event, Dr. Ushma Upadhyay, Associate Professor at Advancing New Standards in Reproductive Health (ANSIRH), advocated a “believe the woman” approach — meaning that the woman, not the abortionist, would bear the burden of determining her child’s gestational age. Watch the video below at 1:30:00:
Abortionist Felicia H. Stewart founded ANSIRH and was previously awarded by the Population Council. ANSIRH publishes abortion workbooks which they promote as an “all-inclusive curriculum with tools to train new abortion providers.” ANSIRH is part of UCSF’s Bixby Center for Global Reproductive Health, and its focus is abortion. Bixby claims its work has “led to new methods of abortion and expanded the abortion care workforce.” The center trains abortion providers though its Ryan Residency Training Program.
In the video Upadhyay says, “Our team, we currently have several innovative trials that we are working on. Our own Dan Grossman has a portfolio of really cutting edge research projects investigating alternative models of provision of medication abortion….” Grossman is behind the push to expand “self-managed” DIY abortion by way of two clinical trials for dispensing via pharmacy (brick and mortar as well as online). He is also an abortion trainer at UCSF, and is on staff as a Senior Advisor at Ibis Reproductive Health. Ibis is funded by the David and Lucile Packard Foundation — an abortion pill investor — which, as recently as January 2020, was also directly funded by abortion pill manufacturer DANCO Laboratories.

Ushma Upadhyay details “no test” abortion
Upadhyay says, “I’m in the process of designing a study to test the feasibility, safety and effectiveness of a telemedicine model of abortion. I’m working with Dr. Karen Meckstroth in our department. And that’s the California Home Abortion by Telahealth study.” (Meckstroth is on staff at UCSF and is the director of Women’s Options Center, which commits abortion up to the 23rd week of pregnancy. It is one of the sites for the pharmacy-dispensed abortion pill clinical trial, along with UC Davis, the University of Washington, Kaiser Permanente, and Planned Parenthood.)

Daniel Grossman Pharmacy abortion pill Clinical Trial sites
Upadhyay continued, “This study would be the first to omit any clinic visits or clinic tests that are usually required. To access whether perspective patients are within the ten week gestational limit for medication abortion, we’re going to do something very revolutionary. We’re going to actually believe people when they say that they’re certain of when their last menstrual period is and calculate that date based that.” (emphasis added)
In 2018, the Society of Family Planning (SFP) awarded a grant of nearly $5 million to Upadhyay for “The California home abortion by telemedicine (CHAT) study.” That study protocol was discussed in November of 2018 at the American Public Health Association (APHA) Annual Meeting:
… Global research has shown that direct-to-patient telemedicine, without routine tests, such as ultrasound, Rh immune globulin screening and human chorionic gonadotropin (hCG) follow up testing, is safe and acceptable. However, a better understanding is needed of patient preferences…. Finally, we will present the protocol for the California Home Abortion by Telemedicine (CHAT) Study, a patient-centered abortion care model offering direct-to patient telemedicine-based abortion, designed to meet individual patient’s needs and desires. This model will offer a no-test telemedicine abortion or referrals to tests as the patient desires.

SFP grant for no test abortion CHAT study in 2018
The APHA summary shows clearly how the purpose of the “no test” abortion pill protocol is to expand abortion — not to reduce risk during COVID-19 — stating, “Results from this study could help U.S. women access abortion care faster and more conveniently while still being clinically supported.” (emphasis added)
It appears that the plan was to test the protocol first; however, COVID-19 provided the abortion industry an immediate opportunity to launch the protocol nationwide.
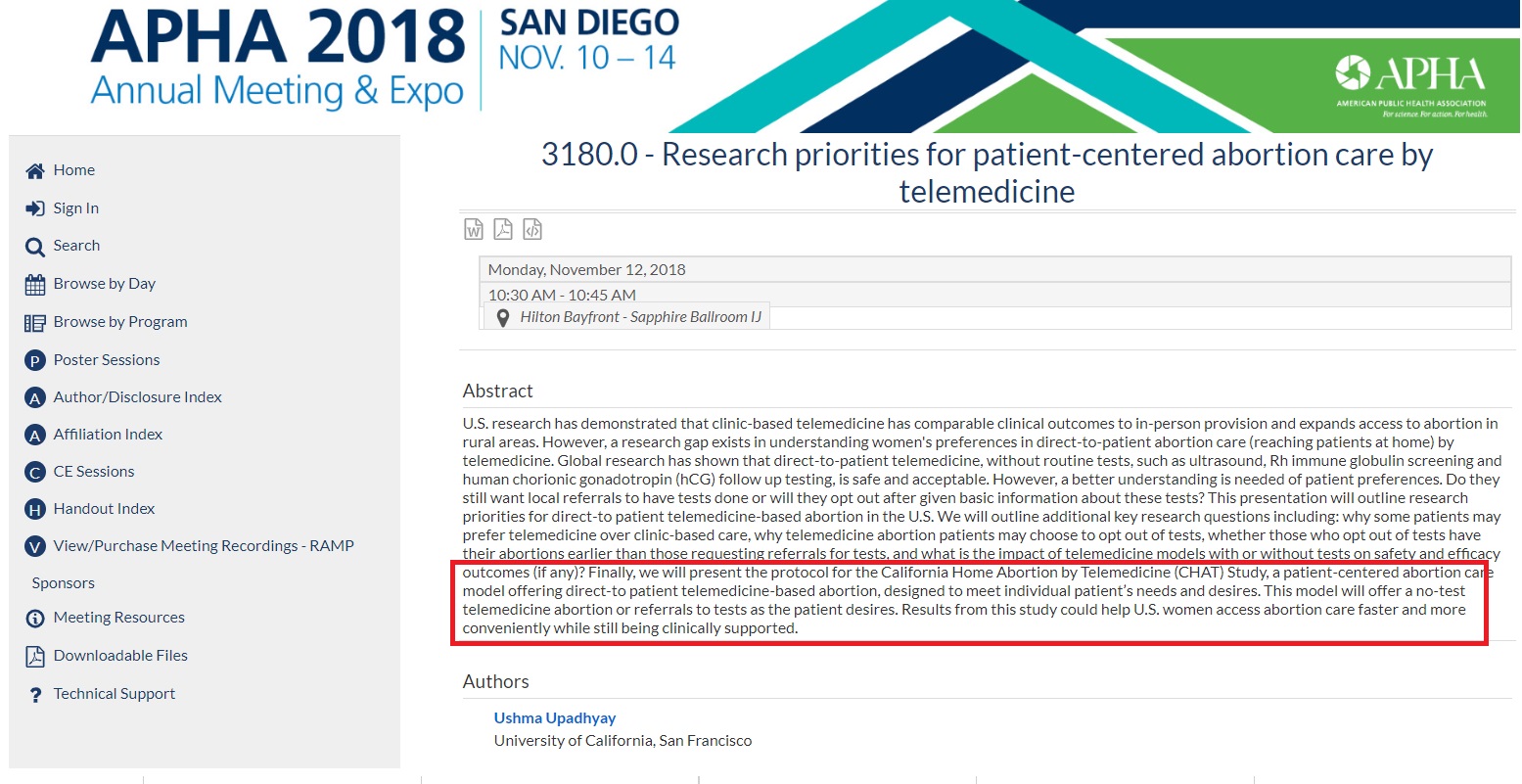
APHA 2018 no test abortion CHAT study summary
Live Action News previously reported that a “no test” abortion pill protocol has been implemented by Maine Family Planning during COVID-19. This abortion business is also part of the Gynuity-sponsored, rapidly expanding TelAbortion clinical trial, which offers the abortion pill to children as young as 10.

Maine FP TelAbortion abortion pill referral recruits ages ten years old
Previously, Live Action News noted:
In the ‘contact-free’ process, women are first screened by phone, and then have a telehealth visit with a clinician. They can then drop by any one of the 18 facilities operated by Maine Family Planning to pick up the pre-packaged medication, along with instructions and a pregnancy test, without ever receiving an in-person consultation or exam. Part of the benefit of this program for the abortion chain is that women can get abortion pills easily, and therefore, the facilities can commit more chemical and surgical abortions…Known complications of the abortion pill include heavy cramping, nausea/vomiting, and hemorrhaging. Women have died from abortion pills, most often from infection and undiagnosed ectopic pregnancies.
Many women who undergo the chemical abortion regimen have reported experiencing pain and trauma as they go through abortion in their homes or dorm rooms, possibly even having to dispose of the bodies of their preborn children themselves. And despite what they’re told by the abortion industry, post-abortive women have said that it is not an easy experience, describing it as terrifying, humiliating, painful, horrific, and like “the scene of a murder.”
In addition to the Bixby panel previously mentioned, there is ample evidence that the “no test” abortion plan was never a response to COVID-19 and was long expected:
- NAF’s 2020 clinical guidelines allow for abortions past FDA’s approved dating of 70 days/10 weeks.
- In 2018, NAF’s clinical guidelines called for Rh testing for “all patients with unknown Rh status” but were updated in 2020, published prior to the COVID-19 outbreak to forego Rh testing… “for women having any type of abortion before 56 days… [and for] those using [the abortion pill] before 70 days LMP….”
- In May 2019, abortionists Alice Mark, Daniel Grossman, advocated removing Rh testing in the Journal Contraception because they believed it to be a “barrier” to abortion access.
- In 2019, Gynuity Health Projects had plans to remove “barriers to abortion access” by eliminating testing and expanding medication abortion into the second trimester.

Gynuity plan to demedicalize abortion by expanding to 11 or 12 weeks
By now it should be clear that the “no-test” abortion protocol was in development long before COVID-19. Dispensing abortion pills without any ultrasounds, blood tests, or definitive determination of gestational age could lead to such great inaccuracies that women may see clearly identifiable body parts of their aborted children during the abortion pill process. While this could no doubt traumatize women, the abortion industry’s overhead costs would fall. This is the true motivation behind fast tracking “no test” abortion pills — not protecting women’s health, and not curbing the spread of COVID-19.
Editor’s Note: FDA has received reports of serious adverse events in women who took mifepristone. As of June 30, 2021, there were reports of 26 deaths of women associated with mifepristone since the product was approved in September 2000, including two cases of ectopic pregnancy (a pregnancy located outside the womb, such as in the fallopian tubes) resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal. The adverse events cannot with certainty be causally attributed to mifepristone because of concurrent use of other drugs, other medical or surgical treatments, co-existing medical conditions, and information gaps about patient health status and clinical management of the patient. A summary report of adverse events that reflects data through June 30, 2021 is here.
“Like” Live Action News on Facebook for more pro-life news and commentary!