Expanding abortion has long been the goal of the industry which profits from it. Abortion pill clinical trials are being conducted and expanded by insiders within the abortion industry — some connected to groups directly funded by the manufacturer of the abortion pill — in an attempt to move closer to this goal. Live Action News has already uncovered several studies of this nature, and now, two more clinical trials have been found. One study is being conducted in the late second trimester on minority girls and women in West Africa. Another is a U.S. mail order by pharmacy trial. Both studies involve groups or individuals connected in some way to the abortion pill manufacturer, Danco Laboratories.
Live Action News has previously documented the following clinical trials:
- Direct-To-Consumer (pills sent via mail after TelAbortion or Telemedicine interview, and here)
- Pharmacy dispensing (and here)
- Non-pregnant women (study has since been withdrawn)
- Study to discredit Abortion Pill Reversal (now suspended)
- Menstrual regulation (and here)
Abortion Pill for Second Trimester Study Outside U.S.
In the U.S., according to the FDA, “Mifeprex is approved, in a regimen with misoprostol, to end a pregnancy through 70 days gestation.” Gynuity Health Projects, which has worked to expand abortion by sponsoring U.S. abortion pill clinical trials, is now recruiting children and adult women outside the U.S. for an experimental abortion pill trial for late abortions. Gynuity was founded by Beverly Winikoff, M.D., M.P.H, who had a previous contract with Danco Laboratories as seen in the image below. Winikoff was previously employed at the Population Council, which brought the abortion pill into the U.S. and set up the pill’s manufacturer, Danco Laboratories, LLC. In addition, Gynuity is funded (see here) by Danco’s original business investor, the Packard Foundation. 
Gynuity founder received funding from abortion pill mfg Danco
Gynuity is also funded by Ibis Reproductive Health (see 2017 990 report), an organization that is directly funded by Danco Laboratories.
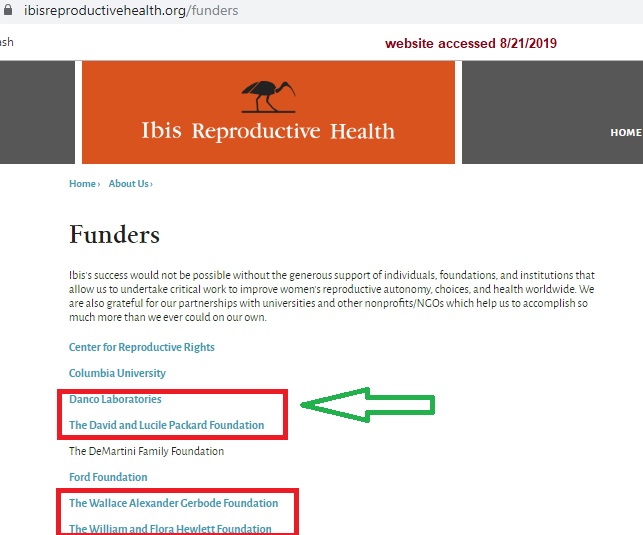
Ibis receives funds from abortion pill Mgf Danco Laboratories and Packard Gerbode Hewlett
Gynuity’s latest clinical trial, “Mifepristone and Misoprostol for 2nd Trimester Termination of Pregnancy in Burkina Faso” expects to “examine the effectiveness and feasibility of a mifepristone [Medabon]-misoprostol [Misoclear] medical abortion regimen in terminating pregnancies 13-22 weeks.” In other words, it will use the abortion pill to attempt late second trimester abortions.
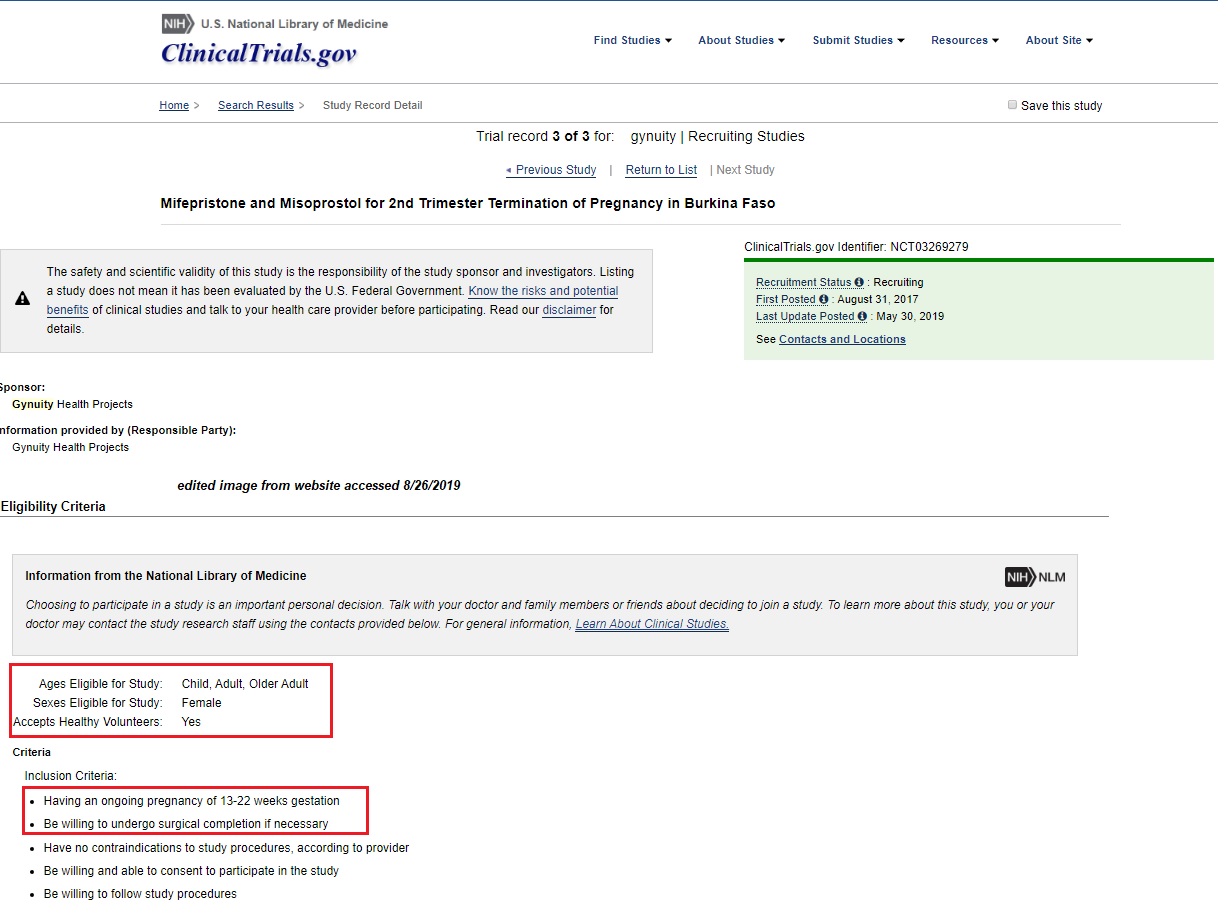
Abortion Pill Clinical Trial outside US for second trimester sponsored by Gynuity (Image: FDA Clinical Trial website)
TelAbortion Clinical Trial Expands:
A TelAbortion study (mail order abortion pill) conducted by Gynuity — described in the trial as “Direct-to-Consumer” — has previously been documented by Live Action News. That trial originally sought to enroll 50 women at two locations. However, as of June 2019, that trial now intends to enroll 1,000 participants with age eligibility as young as 10 years old. The age of recruitment is concerning, given past abuses by abortion facilities and Planned Parenthood centers’ failure to report child sexual abuse, along with potential violations of parental consent and notification laws in certain states.
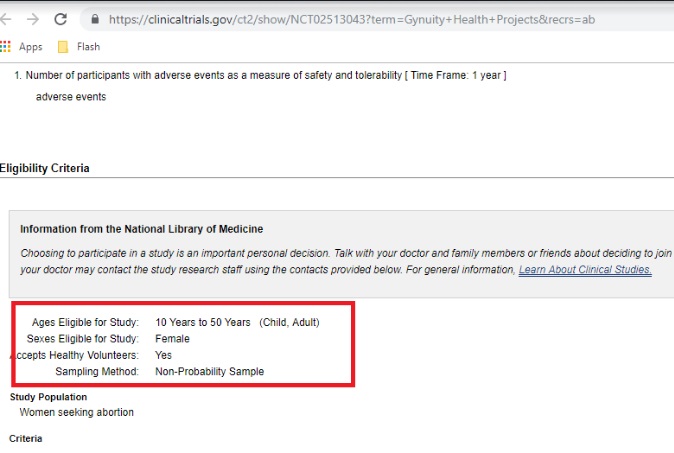
Feasibility TelAbortion clinical trial ages 10 to 50 accessed June 20 2019
Mail Order by Pharmacy Clinical Trial:
A new clinical trial is being launched by Dr. Daniel Grossman, which will seek to offer abortion pills by “dispensing mifepristone via a mail-order pharmacy.” The study, entitled “Mail Order Mifepristone Study” or “Feasibility and Acceptability of Dispensing Mifepristone Via Mail Order Pharmacy,” has been described as a “a prospective cohort study of patients obtaining medication abortion via mifepristone dispensed from an online mail order pharmacy.” The study is sponsored by the University of California, San Francisco (UCSF) and plans to recruit females who are “15 years and older.”
UCSF is a known abortion trainer recently exposed for their experimental use of aborted fetal tissue studies on animals. Live Action News previously documented how UCSF has been pushing for the expansion of abortion via the abortion pill and pushing for the lifting of important safety requirements — REMS — put in place by the FDA.
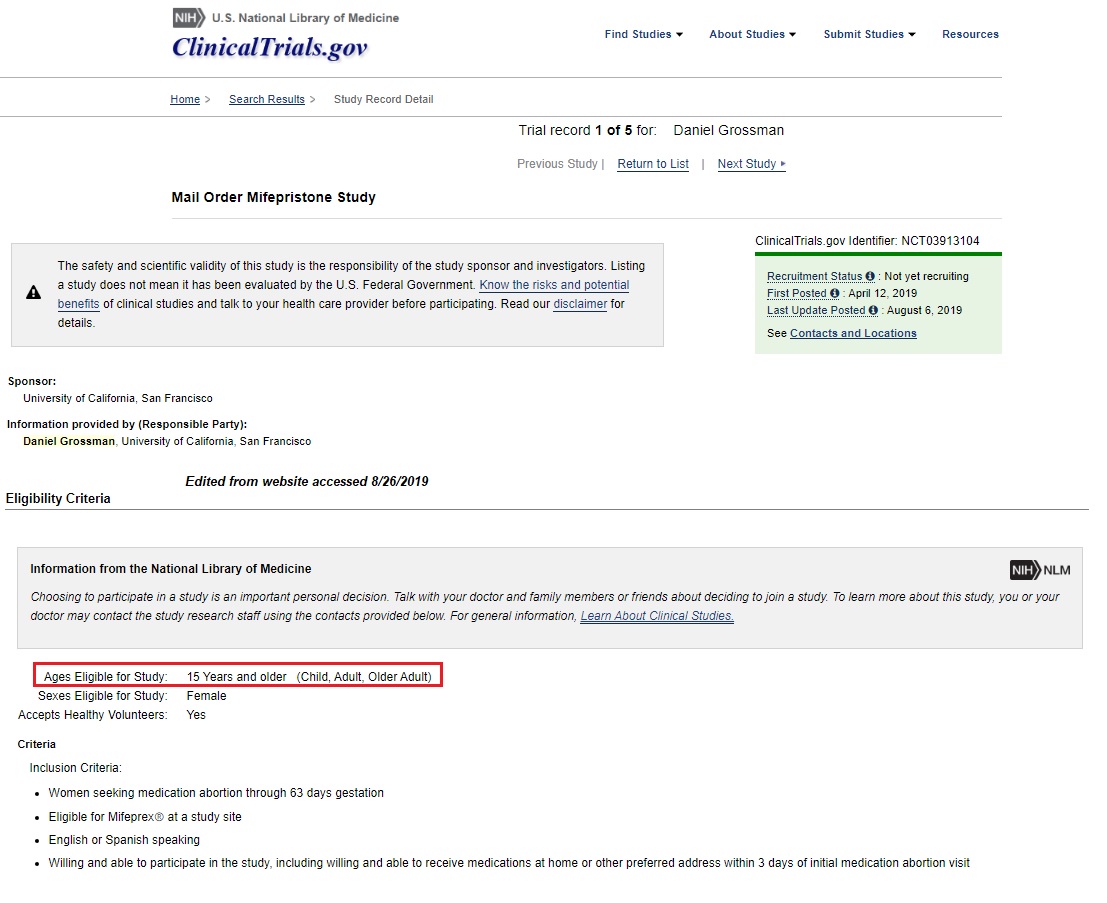
Abortion pill trial by Pharmacy Mail Order conducted by UCSF and Daniel Grossman
Investigators say they will recruit patients at “10-15 primary care and internal medicines sites not currently providing abortion services around the country.” The sponsor states, “Patients will go through routine in clinic visits to determine eligibility and then will receive the medications in the mail at their preferred address. The investigators will survey patients 3 and 14 days after the initial clinic visit and interview providers at the end of the study.”
Principal investigator Dr. Daniel Grossman is behind a separate trial for pharmacy dispensing, previously mentioned above. What is not mentioned in either trial is that Grossman is senior adviser at Ibis Reproductive Health, an organization directly funded by Danco Laboratories. According to a job post published by ANSIRH, a program of the Bixby Center for Global Reproductive Health at UCSF, the group is hiring a “project coordinator” to assist Grossman, who became director of ANSIRH in 2015.
Daniel Grossman works with Ibis funded by abortion pill mfg Danco
Expansion Expected
As with the previously mentioned TelAbortion study, this mail order by pharmacy trial is also set up to expand “[a]fter an initial pilot test with 25 patients, the investigators will identify 15-20 clinics and/or medical facilities not currently providing medication abortion to participate in the study. The study team will train primary care providers to provide medication abortion with the medications dispensed via mail order pharmacy. The investigators aim to recruit approximately 400 patients for this study across all the sites….”
These clinical trials are a work around current FDA safety requirements which require the abortion pill regimen to be dispensed by a certified prescriber and not online or sent through the mail. Abortion enthusiasts like Grossman and Gynuity are testing the FDA safety system, and instead of ending their trials they continue expanding them into different states or agencies, thus indefinitely dispensing the abortion pill regimen outside approved FDA protocols. These groups then publish their “findings” in studies funded by original investors of Danco — such as the Buffett Foundation and the Packard Foundation, which then claim the expansion of abortion is safe.
The conflicts are glaring.
“Like” Live Action News on Facebook for more pro-life news and commentary!







