With the spotlight on the abortion pill (mifepristone) as the courts wrangle over the legitimacy of the U.S. Food and Drug Administration’s original approval of the drug, Live Action News has put together a timeline on the process from its beginnings to today. An estimated 5.6 million human lives have been ended through the use of this drug, which also comes with potentially serious side effects for women.
Background
The abortion pill (mifepristone) is sold in the United States under the brand name Mifeprex and was originally invented and patented in 1980 by the French pharmaceutical company Roussel-Uclaf, a subsidiary of Germany’s Hoechst AG. It was approved for sale in the United States in September of 2000, with specific restrictions that it not be shipped directly to women but be administered by certified prescribers at a clinic or hospital where follow-up care would be available.
Timeline
PRE-APPROVAL (1994): In 1994, with the encouragement of the Clinton administration, Roussel-Uclaf assigned the U.S. rights of marketing and distribution of the abortion pill (known then as RU-486) to the eugenics-founded Population Council. The right to distribute the drugs was later handed over to Danco Laboratories, a sub-licensee of the Population Council. Then, by 1996, the Population Council (funded in part with investments from the Buffett and Packard Foundations) submitted its application for the drug to the FDA, and a series of clinical trials began.
U.S. APPROVAL (2000): In 2000, following those clinical trials, the U.S. FDA approved mifepristone as an abortion pill for use up to 7 weeks of pregnancy in a regimen along with the drug misoprostol.
SAFETY RULES ADDED (2011): In 2011, the FDA made the decision to place the drug under its REMS safety system, but not before multiple women had died in association with use of the abortion pill regimen.
OBAMA ADMIN WEAKENS FDA SAFETY RULES (2016): Just five years later, in 2016, the Obama administration FDA weakened the REMS by removing the requirements that women or teen girls take the first drug in front of a clinician, in-person at the location of a certified prescriber and that the manufacturer report the drug’s non-fatal adverse events (complications). The drug’s allowed use was also extended for use on preborn children up to 10 weeks (70 days) of pregnancy.
GENERIC MANUFACTURER APPROVED (2019): In 2019, the FDA approved GenBioPro to become the generic manufacturer of mifepristone.
ABORTION INDUSTRY ‘NO-TEST’ PROTOCOL (2020): In 2020, the abortion industry rolled out a ‘no-test‘ abortion pill protocol which removed important labs, testing, and blood work needed to accurately date a pregnancy and rule out deadly ectopic pregnancies before administering the abortion pill. Rapid expansion of the abortion pill continued in the ensuing years, even as the abortion industry openly flouted FDA gestational limits and safety regulations, even encouraging women to lie about abortion pill complications.
BIDEN ADMIN WEAKENS SAFETY RULES (2021): In April of 2021, under the guise of the COVID-19 pandemic, the Biden administration FDA temporarily enabled abortion pill distribution and expanded the REMS to limited mail-order pharmacy distribution. By December of 2021, the Biden FDA had further weakened the REMS by eliminating the in-person dispensing requirement and enabling the abortion pill to be permanently shipped by mail.
LAWSUIT CHALLENGES FDA APPROVAL (2022): The following year (2022) saw key a legal challenge, when in November, the Alliance Defending Freedom filed a federal lawsuit on behalf of the Alliance for Hippocratic Medicine, challenging FDA approval of mifepristone. The move followed the FDA’s unsatisfactory responses to a number of citizen petitions the group had submitted. That same year, GenBioPro (GBP) voluntarily dismissed a lawsuit challenging pro-life legislation in Mississippi.
BIDEN ADMIN FURTHER WEAKENS SAFETY RULES (2023): In January 2023, the Biden FDA further gutted the REMS by announcing it would allow retail pharmacies to dispense the drug. It was at this time that the FDA officially updated the drug’s Post-Marketing Adverse Events Summary to state that “As of June 30, 2022, there were 28 reports of deaths* in patients associated with mifepristone since the product was approved in September 2000…” The FDA also documented that between 2000 and June 2022, the abortion pill had ended the lives of 5.6 million preborn babies, including over 500,000 in 2020 alone.
Pro-abortion politicians filed a competing lawsuit (State of Washington v. FDA) in February, requesting that the judge enjoin the FDA from (1) enforcing or applying the 2023 REMS, and (2) taking any action to remove mifepristone from the market or otherwise cause the drug to become less available…”
By April 7, 2023, U.S. District Court Judge Matthew J. Kacsmaryk had ruled in favor of Alliance for Hippocratic Medicine to suspend the 2000 approval and subsequent FDA changes to the abortion pill. On the same day, U.S. District Judge Thomas O. Rice granted pro-abortion politicians in the Washington State case a preliminary injunction to bar the FDA from “altering the status quo and rights as it relates to the availability of Mifepristone under the current operative January 2023 Risk Evaluation and Mitigation Strategy under 21 U.S.C. § 355-1 in Plaintiff States.” Just a few days later on April 12, the U.S. Court of Appeals for the Fifth Circuit issued a partial stay in Alliance for Hippocratic Medicine. While the circuit panel did not suspend the drug’s 2000 approval, the decision upheld the suspension of mail-order abortion pills and reinstated safety requirements dated prior to 2016.
Meanwhile, the Biden Administration intends to ask the United States Supreme Court to intervene. As the nation awaits future court rulings, the abortion industry is already pivoting to a less effective and unapproved one-drug regimen of misoprostol only.
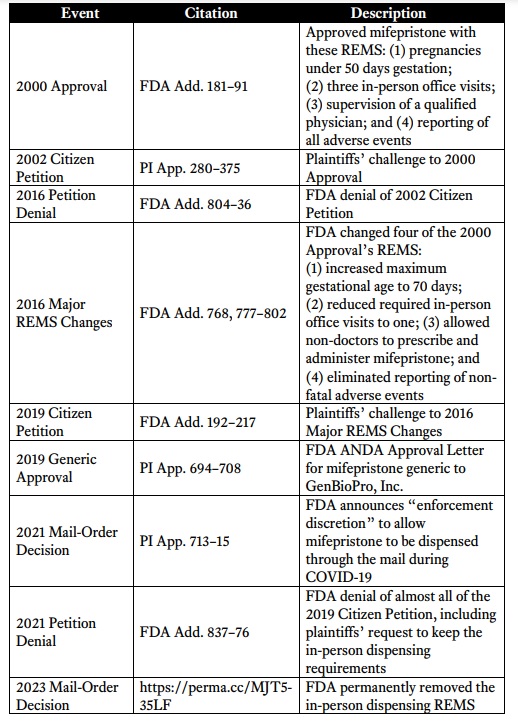
Abortion pill changes 2000 to 2023 from AHM v FDA appeal
*THE FDA HAS RECEIVED REPORTS OF SERIOUS ADVERSE EVENTS IN PATIENTS WHO TOOK MIFEPRISTONE. AS OF JUNE 30, 2022, THERE WERE 28 REPORTS OF DEATHS IN PATIENTS ASSOCIATED WITH MIFEPRISTONE SINCE THE PRODUCT WAS APPROVED IN SEPTEMBER 2000, INCLUDING TWO CASES OF ECTOPIC PREGNANCY (A PREGNANCY LOCATED OUTSIDE THE WOMB, SUCH AS IN THE FALLOPIAN TUBES) RESULTING IN DEATH; AND SEVERAL FATAL CASES OF SEVERE SYSTEMIC INFECTION (ALSO CALLED SEPSIS). THE ADVERSE EVENTS CANNOT WITH CERTAINTY BE CAUSALLY ATTRIBUTED TO MIFEPRISTONE BECAUSE OF CONCURRENT USE OF OTHER DRUGS, OTHER MEDICAL OR SURGICAL TREATMENTS, CO-EXISTING MEDICAL CONDITIONS, AND INFORMATION GAPS ABOUT PATIENT HEALTH STATUS AND CLINICAL MANAGEMENT OF THE PATIENT. A SUMMARY REPORT OF ADVERSE EVENTS THAT REFLECTS DATA THROUGH JUNE 30, 2022, IS HERE. THE FDA HAS REVIEWED THIS INFORMATION AND DID NOT IDENTIFY ANY NEW SAFETY SIGNALS. THE FDA INTENDS TO UPDATE THIS SUMMARY REPORT AS APPROPRIATE.