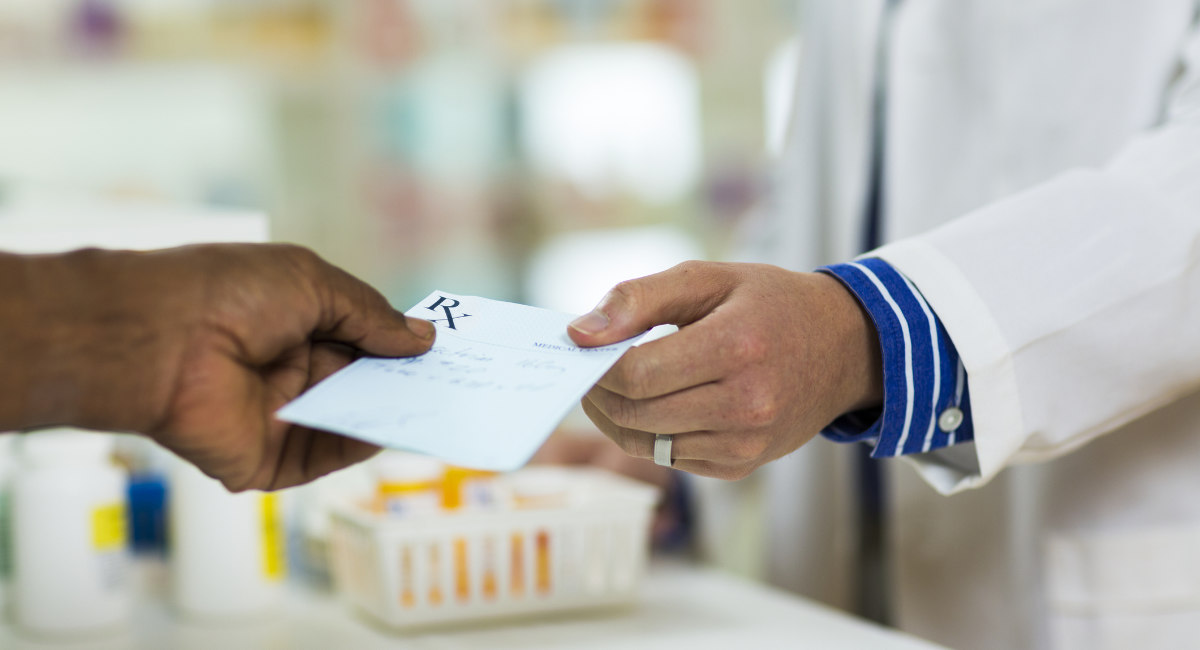
The abortion pill can now be sold through retail pharmacies with a prescription, according to the U.S. manufacturer of the abortion pill, Danco Laboratories. The change made on January 3, 2023, was not yet published by the U.S. Food and Drug Administration (FDA) when it grabbed the attention of multiple media outlets due to an announcement released by Danco.
“Pharmacies who become certified in the Mifepristone REMS Program may dispense Mifeprex® directly to patients upon receipt of a prescription from a certified Mifeprex® prescriber, provided a Prescriber agreement is provided or on file with the certified pharmacy,” Danco’s press release states.
REMS is a safety requirement put in place by the FDA to monitor a limited number of drugs with specific concerns.
“Danco has worked to ensure that the REMS modifications will not disrupt the medication abortion services that are currently being provided by existing Mifeprex providers whether through clinics, medical offices, hospitals or telehealth utilizing our mail order pharmacies, American Mail Order Pharmacy (AMOP) and Manifest Pharmacy. We expect that there may be an adjustment period for Mifeprex® providers as we implement the changes to the REMS. Current providers will be able to continue provision of Mifeprex under their current Prescriber Agreements for a number of months before an updated Prescriber Agreement must be put in place,” Danco also wrote.
In response, Susan B. Anthony Pro-life America pointed out that “[m]ultiple peer-reviewed studies confirm that women are at risk of severe side effects after taking chemical abortion pills, including hemorrhaging, the need for follow-up surgery, and even death. Studies have found that chemical abortion has four times the complication rate of surgical abortion, and these risks only increase with advanced pregnancy and lack of medical supervision. Peer-reviewed research from Charlotte Lozier Institute has also found that after a chemical abortion there is a 53% greater risk for an ER visit for abortion complications than after a surgical abortion.”
An update to the FDA’s website came later in the day, and states, “As of June 30, 2022, there were 28 reports of deaths in patients associated with mifepristone since the product was approved in September 2000, including two cases of ectopic pregnancy (a pregnancy located outside the womb, such as in the fallopian tubes) resulting in death; and several fatal cases of severe systemic infection (also called sepsis).”*
Will retail pharmacies agree to dispense abortion drugs?
“For the first time, retail pharmacies, from corner drugstores to major chains like CVS and Walgreens, will be allowed to offer abortion pills in the United States under a regulatory change made Tuesday by the Food and Drug Administration. The action could significantly expand access to abortion through medication,” New York Times reported.
In February of 2022, a Bloomberg writer claimed that Walgreens Boots Alliance Inc. wasn’t seeking pharmacy certification, but noted at the time that CVS Health Corp. would not comment on whether it planned to do so. It is unknown if that will change.
Pharmacy Agreement
The Pharmacy Agreement states, “Pharmacies must designate an authorized representative to carry out the certification process and oversee implementation and compliance with the Mifepristone REMS Program on behalf of the pharmacy. Healthcare settings, such as medical offices, clinics, and hospitals, where mifepristone will be dispensed by or under the supervision of a certified prescriber in the Mifepristone REMS Program do not require pharmacy certification.”
The pharmacy is required to, among other things:
- Report any patient deaths to the prescriber… and remind the prescriber of their obligation to report the deaths to Danco Laboratories, LLC.
- Not distribute, transfer, loan or sell mifepristone except to certified prescribers or other locations of the pharmacy.
- Maintain records of Prescriber Agreement Forms, dispensing and shipping, and all processes and procedures including compliance with those processes and procedures.
- Maintain the identity of Mifeprex patients and prescribers as confidential and protected from disclosure except to the extent necessary for dispensing under this REMS or as necessary for payment and/or insurance.
- Train all relevant staff on the Mifepristone REMS Program requirements.
- Comply with audits carried out by the Mifepristone Sponsors or a third party acting on behalf of the Mifepristone Sponsors to ensure that all processes and procedures are in place and are being followed.
Allowing for over-the-counter distribution of the most dangerous form of abortion is horrifying! The @FDA needs to restore their Risk Evaluation & Mitigation Strategies that, at minimum, protected women. Sign the petition to hold the FDA accountable! https://t.co/glTsUwXPR3 pic.twitter.com/qBZ082SDeA
— Kristan Hawkins (@KristanHawkins) January 4, 2023
Pharmacist studies show problems
Studies published by Dr. Daniel Grossman, an abortionist and recent National Abortion Federation board member who has served as a consultant to Planned Parenthood Federation of America and the Center for Reproductive Rights (along with leadership in other pro-abortion groups), revealed glaring issues with retail pharmacy dispensing of the abortion pill.
Despite multiple conflicts of interest, Grossman has co-authored abortion pill studies potentially used by FDA to weaken the REMS. His ethics on the topic were recently called into question when he suggested that emergency healthcare personnel should leave important medical facts out of the records of women presenting with abortion pill complications post-Roe.
In June of 2021, Grossman and his colleagues (three of whom were consultants for Mifeprex manufacturer Danco, Inc. and the generic manufacturer GenBioPro), published data from one survey on “pharmacists’ knowledge, perceptions, and experiences dispensing mifepristone” from six clinical sites in California and in Washington state.
As Live Action News previously reported, those results revealed a lack of understanding among pharmacists about risks associated with the abortion drug.
The report showed that barely half of pharmacists (51%) understood the percentage of patients requiring blood transfusions, 53% understood the risks of taking mifepristone, 53% understood minor consent laws for abortion. Just one-third (30%) understood adverse effects such as fever and chills.
A second report, “‘No Big Deal’: A Qualitative Study of Pharmacists’ Perspectives on Dispensing Mifepristone for Medication Abortion,” utilizing the same study, was published in July of 2022. It concluded that “most pharmacists supported dispensing mifepristone.” In this study, authors Sally Rafie and Karen Meckstroth also admitted conflicts of interest. As Live Action News previously documented, both have known financial ties to either Danco or GenBioPro.
In addition, the study sites included the University of California where abortionists are trained, and it noted that only a small number of pharmacists who participated were even questioned on follow-up. Still, some pharmacists expressed concern with dispensing abortion pills.
Background
The January 3, 2023, move follows changes issued last year by the FDA to weaken the REMS by eliminating the in-person dispensing requirement and enabling the abortion pill to be shipped by mail. Prior to that decision, the FDA’s REMS prohibited the abortion pill from being dispensed online or in pharmacies and required the pill to be dispensed in person in approved clinics or hospitals that could properly date a woman’s pregnancy, rule out life-threatening ectopic pregnancies, and offer or refer for emergency care in cases of complications or when abortion pill failures result in incomplete abortions.
The December 16, 2021 modification removing the in-person requirement was posted to the Mifeprex Q&A page where the FDA was vague (at that time) on whether their changes would permit brick and mortar pharmacies to directly dispense the abortion pill. Modification proposals were to be submitted to the FDA by the pill’s manufacturers Danco Laboratories and (generic) GenBioPro.
A January 2022 e-mail response from the FDA to Live Action News indicated the FDA did not yet have sufficient data to warrant pharmacy dispensing of the drug.
“At this time, we don’t have data to determine whether mifepristone for medical termination of early pregnancy can be dispensed safely through retail pharmacies in accordance with the REMS,” the FDA told Live Action News (emphasis added). Then, an August 12 e-mail from the FDA to Live Action News indicated that the process to determine pharmacy distribution had not been fully approved. “FDA will promptly review the REMS modification submissions made by the applicants; once any submissions are approved, the REMS modifications will be effective,” the email read (emphasis added).
Yet in July of 2022, prior to the publication of the pharmacy certification process, the Biden administration sent out a letter essentially threatening pharmacies who “refuse” to fill prescriptions for mifepristone/Mifeprex.
Live Action president and CEO Lila Rose reacted to the news in a press release, saying:
It’s absolutely disgusting that your local pharmacist will now be dispensing poison that ends an innocent human life alongside antibiotics and allergy medication… This news is tragic not only for the innocent victims of abortion, but also for their mothers. The highest priority for the new pro-life majority in the U.S. House of Representatives must be to stop the deadly abortion pill and rein in the reckless Biden Administration and their lackeys in the FDA.
In 2020, abortion pills took the lives of nearly 500,000 preborn babies while sales from the abortion pill regimen now make up an astounding 53% of nationally reported abortion numbers. Numbers published by the FDA through June of 2021 revealed that the abortion pill had ended (at that time) the lives of nearly 5 million preborn human beings since its approval in 2000.
*The FDA has received reports of serious adverse events in patients who took mifepristone. As of June 30, 2022, there were 28 reports of deaths in patients associated with mifepristone since the product was approved in September 2000, including two cases of ectopic pregnancy (a pregnancy located outside the womb, such as in the fallopian tubes) resulting in death; and several fatal cases of severe systemic infection (also called sepsis). The adverse events cannot with certainty be causally attributed to mifepristone because of concurrent use of other drugs, other medical or surgical treatments, co-existing medical conditions, and information gaps about patient health status and clinical management of the patient. A summary report of adverse events that reflects data through June 30, 2022, is here. The FDA has reviewed this information and did not identify any new safety signals. The FDA intends to update this summary report as appropriate.
Editor’s Note, 1/6/23: This article has been updated with a press release link and comments.