The abortion pill has ended the lives of nearly 5 million babies in the U.S. since its approval in 2000, according to updated data published by the U.S. Food and Drug Administration (FDA). And in just the past three years, two additional deaths have been attributed to the abortion pill.
The abortion pill, mifepristone (brand name Mifeprex), was approved by the FDA in 2000 for the termination of pregnancy in a regimen along with a second drug, misoprostol.
In December of 2018, the FDA reported 24 deaths associated with abortion pills since its approval but on December 16, 2021 the FDA published an updated “Post-Marketing Adverse Events Summary” revealing that as of June 30, 2021, that number has now climbed to 26. The report also tragically reveals that the “estimated number of women who have used [abortion pill] mifepristone in the U.S. for medical termination of pregnancy through the end of June 2021 is approximately 4.9 million women.”
Just last week, the Biden-Harris FDA weakened important safety regulations (REMS) on abortion pills, eliminating the in-person requirement and permanently allowing the deadly drug regimen to be shipped by mail. REMS ,Risk Evaluation and Mitigation System, is a “drug safety program that the… FDA can require for certain medications with serious safety concerns.”
The FDA wrote, “It is not uncommon for FDA to receive reports of serious adverse events for prescription drugs after they are approved. FDA has received reports of serious adverse events in women who took mifepristone. As of June 30, 2021, there were reports of 26 deaths of women associated with mifepristone since the product was approved in September 2000, including two cases of ectopic pregnancy (a pregnancy located outside the womb, such as in the fallopian tubes) resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal.”
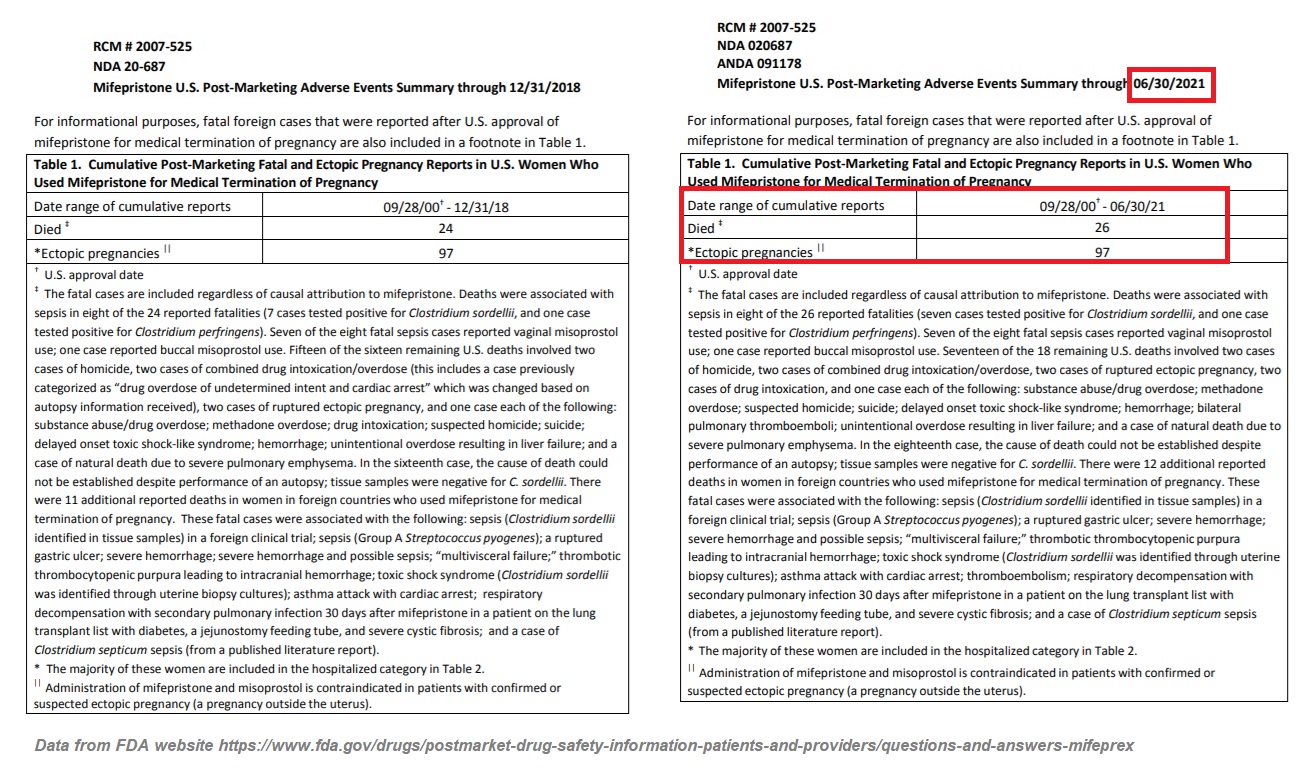
Abortion pill adverse events reports of deaths for Mifepristone by FDA through June 2021
According to the FDA, “These events cannot with certainty be causally attributed to mifepristone because of information gaps about patient health status, clinical management of the patient, concurrent drug use, and other possible medical or surgical treatments and conditions.”
“The FDA has reviewed this information and did not identify any new safety signals,” the website stated. “The FDA intends to update this summary report on an annual basis or as appropriate.”
Abortion pill-related deaths began shortly after approval
The Population Council, a eugenics-founded “non-profit,” brought the abortion pill (known then as RU-486) into the U.S. and later set up the pill’s manufacturer, Danco Laboratories, LLC, a sub-licensee of the Population Council. The FDA’s abortion pill approval process, which culminated in 2000, has been highly secretive as Live Action News has documented. Tragically, shortly after approval, deaths were reported, and over the ensuing years, abortion pills deaths climbed. Read more about this tragic history here.
In May 2020, Columbia University journalists Lauren Mascarenhas and Abigail Brone interviewed early abortion pill advocates including Dr. Beverly Winikoff, who was employed at the time by the Population Council as the Program Director for Reproductive Health — and was the person in charge of the abortion pill’s clinical trials.
In a stunning admission to these journalists, Winikoff credited the 9/11 terrorist attacks with ‘saving’ the abortion pill, after news of the attack overshadowed the news of a woman’s death from the abortion pill. In 2003, Winikoff left the Population Council to found Gynuity Health Projects, which is heavily funded by abortion pill investors. Under Winikoff’s direction, Gynuity sponsored abortion pill clinical trials, including experiments on African women and a TelAbortion trial willing to mail abortion drugs to girls as young as 10.
Adverse Events/Complications
In April of 2011, the FDA reported over 2,200 adverse effects associated with the abortion pill. At the same time, post-marketing reports showed 14 women had died, 612 were hospitalized, 339 women had experienced blood loss which required transfusions, and several hundred reported cases of infection, some severe.
It was in this year that the FDA determined that a REMS was necessary for mifepristone.

Abortion Pills placed under REMS in 2011 (FDA REMS defined as of 6/3/2020)
Despite the increasing adverse events reports, under 2016 changes put in place by the Obama administration’s FDA, the FDA no longer mandated abortion pill manufacturer Danco Laboratories or GenBioPro (generic manufacturer) notify the FDA of complications (adverse events) other than death.
Still, by December of 2018, the data showed that abortion pill complications had risen again and had caused nearly 4,200 adverse effects, including 1,042 hospitalizations, nearly 600 instances of blood loss requiring transfusions, and other serious complications.
Now, according to the June 30, 2021 update (image below), a handful of adverse events were added to the report, showing that mifepristone had caused 4,207 adverse effects, including 1,045 hospitalizations and 603 instances of blood loss requiring transfusions. The FDA also recorded 413 infections, with 70 being categorized as “severe based on medical review of the available case details.”
“Severe infections generally result in death or hospitalization for at least 2-3 days, require intravenous antibiotics for at least 24 hours and total antibiotic usage for at least 3 days, or have other physical or clinical findings, laboratory data, or surgery that suggest a severe infection,” the FDA also wrote.
But because there is now no requirement to report adverse events other than death, it is unlikely that we can know the true number of injuries from the abortion pill.
No national requirements to report abortion complications exist.
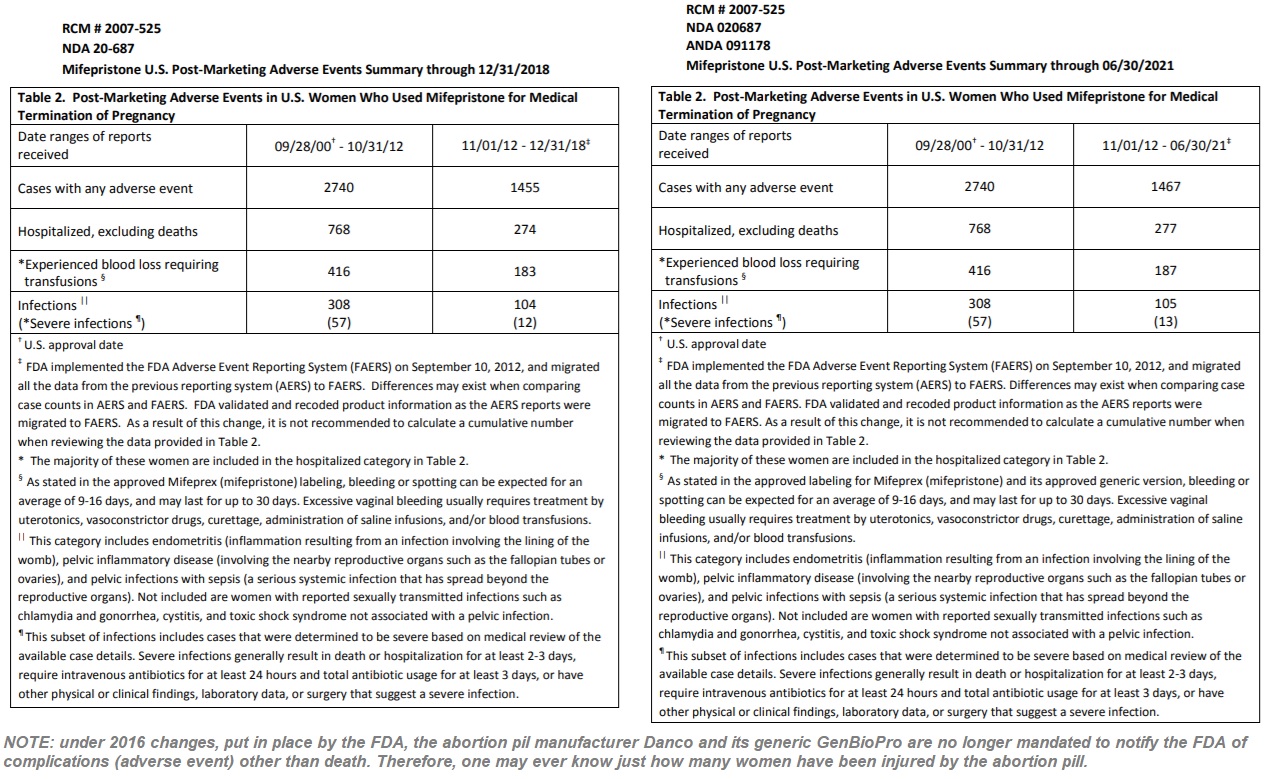
Abortion pill adverse events reports for Mifepristone by FDA through June 2021
The Charlotte Lozier Institute (CLI) recently noted that “an analysis of the data collected under this reporting requirement suggests that a significant number of complications were unreported and that the FDA may have missed as many as 95% of serious chemical abortion adverse events” (emphasis added).
CLI’s executive director, Stephen Billy claimed in a statement that by weakening the REMS, the Biden FDA had cherry-picked “flawed data to give the abortion industry a Christmas gift.”
Complication concerns
A recent analysis of adverse events reports (AERs) submitted to the FDA by the abortion pill manufacturer revealed that abortion pill clients experiencing complications are more likely to receive care from emergency centers than the abortion facilities where they obtained the pills. In many instances, the abortion industry has advised women to visit the emergency room claiming to be experiencing a natural miscarriage, skewing complication reports.
Results of the TelAbortion study, which implemented extremely stringent safety requirements than would be seen in normal use, revealed that 6% of “known outcomes” from the abortion pill were severe enough to result in emergency room or urgent care visits. That number appears to mirror data from the UK.
In the U.S., potentially 20,380 women per year are seeking care at ERs or urgent care facilities after taking the abortion pill.
In addition, a recent study that analyzed Medicaid data within the 17 states currently allowing taxpayer-funded abortions found that the rate of abortion-related ER visits for chemical abortions increased 507% from 2002-2015.
According to the Charlotte Lozier Institute (CLI), the study also found that “[o]ver 60% of abortion-related ER visits following a chemical abortion in 2015 were miscoded as treatment for a miscarriage.” (emphasis added)
Tessa Longbons, senior research associate at CLI, noted in a statement, “The FDA is putting women and girls at considerable risk through regulatory malpractice. The FDA claims the abortion pill is safe, yet peer-reviewed research confirms a 500% increase in the rate of chemical abortion-related emergency room visits. The FDA claims that complications are rare, yet peer-reviewed research from the United States, Finland, and Sweden confirms the abortion pill has a much higher complication rate than surgical abortion.”
“Like” Live Action News on Facebook for more pro-life news and commentary!