Abortion industry insiders insist that there is a large public interest in expanding distribution of the abortion pill by lifting safety requirements for the drug regimen. Now, right on cue, a study done by abortion industry insiders and funded by a large investor of the abortion pill’s manufacturer purports to show that women want “alternative models” for obtaining the abortion pill. But the move is anything but organic and is being strategically driven by abortion industry insiders.
Published by the Journal Contraception, the study claims that women support obtaining abortion pills “(1) in advance from a doctor for future use, (2) over-the-counter (OTC) from a drugstore and (3) online without a prescription.” But there is more beneath the surface at play here. Not only are the Journal and its authors deeply embedded in the abortion industry, but the funding for this study also came from the David and Lucile Packard Foundation, a major investor in Danco, the manufacturer of the abortion pill.
In 1998, according to reports, Packard seeded Danco with $10 million, and Packard also currently funds Gynuity Health Projects, which conducts abortion pill clinical trials. Packard’s investment in Danco included a $14 million loan as early as 1996 to bring the drug to the US, as well as additional grants made in 2000, 2004, and 2009. And yet, the Journal claims there are no conflicts of interest.

Packard Foundation self managed abortion pill study (Image: 2018 study from Journal Contraception)
Additional investors in Danco include George Soros (Open Society), Warren Buffet (Buffet Foundation) and the California-based Kaiser Family Foundation.

Packard Foundation invested in abortion pill manufacturer DANCO (Image: David and Lucile Packard Foundation )
READ: Abortion industry’s own study predictably claims abortion laws not needed
Journal Contraception
- Contraception is the official journal of the Association of Reproductive Health Professionals (ARHP) and the Society of Family Planning (SFP). ARHP was originally founded as The American Association of Planned Parenthood Physicians in 1963 by Planned Parenthood president, Alan Guttmacher, and has gone through multiple name changes.
- The current editor is abortionist Dr. Carolyn Westhoff, a former director at ARHP, who was involved in the clinical trials of RU-486 and previously testified in favor of keeping partial-birth abortion legal. According to SFP, Westhoff served on the Board of the Alan Guttmacher Institute and Planned Parenthood. Westhoff is the Planned Parenthood medical advisor seen on undercover Center for Medical Progress recordings discussing how the aborted baby body parts she provides for research are “fresh.”
- The Journal’s editorial board has been stacked with abortion industry insiders, including a National Abortion Federation board member and members of the Population Council (responsible for bringing the abortion pill into the US and forming DANCO, the manufacturer of the pill).
Authors

- Author Antonia Biggs is a researcher at Advancing New Standards in Reproductive Health (ANSIRH). ANSIRH was founded by abortionist Felicia H. Stewart, previously awarded by the Population Council. Stewart also served on the executive committee of the ARHP, boards of NARAL and the National Abortion Federation and was medical director and staff physician at Planned Parenthood facilities in California. ANSIRH publishes workbooks on abortion training they call an “all-inclusive curriculum with tools to train new abortion providers” and is part of UCSF’s Bixby Center for Global Reproductive Health. The Bixby Center prides itself on abortion as a focus and claims that their work has, “led to new methods of abortion and expanded the abortion care workforce.” Bixby trains abortion providers though its Ryan Residency Training Program.
- Author Sarah Raifman is also associated with ANSIRH and previously worked for the Population Council. She claims abortion is “part of reproductive healthcare,” and supported the idea of public colleges passing out the abortion pill.
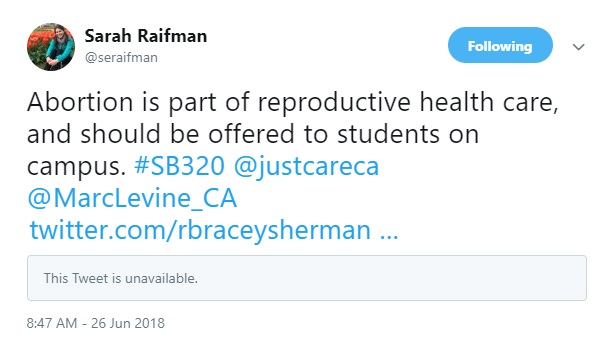
Author Sarah Raifman Tweet June 2018 SB320 (Image: Twitter)
- Author Diana G. Foster is another ANSIRH staffer who opposes late-term abortion restrictions. She sits on the board of the Later Abortion Initiative (LAI), a group with the mission of “increas[ing] the number of sites where later abortion is available” and “expand[ing] the number of physicians who can perform later abortion, especially at 20 weeks’ gestation and beyond.” Foster has been applauded by the abortion advocacy group, NARAL.
Author Diana G Foster applauded by NARAL (Image: Tweeter)
- Author Daniel A. Grossman, MD, is conducting clinical trials for medication abortion dispensed by pharmacists. He worked with the Population Council and testified before a U.S. District Court in 2014 that he provides abortions as a consultant to Planned Parenthood Shasta Pacific. He also serves as a liaison member of the PPFA National Medical Committee and has served for years on the board of NARAL Pro-Choice America.
READ: Amazing: Over 500 babies saved thanks to abortion pill reversal
The abortion pill regimen, RU486, is made up of two drugs: Misoprostol and Mifeprex. This regimen is currently regulated by the FDA under a system known as REMS. As Live Action News previously explained, taking the abortion pill regimen isn’t just a simple thing — if a woman is too far along or if her pregnancy is ectopic, these factors put women at additional risk. There have been 22 reported deaths and thousands of hospitalizations since the abortion pill’s approval in 2000.
Live Action News has previously documented how:
- Abortion insiders brought RU486 to the US.
- Abortion insiders are conducting clinical trials for “home-use” and “self managed” abortions.
- Abortion insiders collaborated to push “home use” abortions.
- Abortion insiders recently coordinated a Tweetfest to promote “self-managed” abortions.
- And now, abortion insiders conveniently roll out a study claiming women support “alternative models of medication abortion provision.”
Despite the clear conflicts of interest surrounding this study about the abortion pill, it and other studies like it are likely to go unchallenged by the mainstream media, just as abortion industry leader Planned Parenthood’s claims continue to go unchallenged. This does a grave disservice to women.
Editor’s Note: FDA has received reports of serious adverse events in women who took mifepristone. As of June 30, 2021, there were reports of 26 deaths of women associated with mifepristone since the product was approved in September 2000, including two cases of ectopic pregnancy (a pregnancy located outside the womb, such as in the fallopian tubes) resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal. The adverse events cannot with certainty be causally attributed to mifepristone because of concurrent use of other drugs, other medical or surgical treatments, co-existing medical conditions, and information gaps about patient health status and clinical management of the patient. A summary report of adverse events that reflects data through June 30, 2021 is here.







