DKT International, which sells millions of abortion pills globally may be shipping abortion drugs into the U.S. manufactured by the Delhi-based Synokem Pharmaceuticals Ltd, which is alleged to have a “shoddy quality record,” according to Bloomberg.com. The outlet also noted that DKT International “has become one of the world’s largest sellers of abortion pills, serving women from India to Mexico.”
According to Bloomberg, “[A]lmost one-fifth of the 30 million products DKT distributes annually for abortions and postpartum hemorrhage prevention come from an Indian company with a record of making substandard medicine.” Bloomberg also alleged:
More than 30 samples of drugs made by Delhi-based Synokem Pharmaceuticals Ltd. — including generic abortion pills… have failed quality tests conducted by Indian regulators and public health researchers since 2018, according to government records and data reviewed by Bloomberg News. The samples contained impurities, lacked the right amount of active ingredient or failed to meet other international standards designed to ensure that medicine is safe and effective, the records show.
In addition, Bloomberg claimed that “[w]hile the inspection reports didn’t flag significant issues, at least 23 samples of Synokem’s drugs have failed quality tests since 2018, according to test results Bloomberg collected from six state and federal drug regulators in India. That’s likely an undercount because most of India’s 36 regional regulators don’t make such results public. One of the tests, involving a misoprostol sample collected at a hospital pharmacy in 2019, had so little active ingredient that a government-run hospital network warned all its members to stop using any medication from the entire batch, official records show,” they wrote.
While Bloomberg claimed that “Synokem abortion pills haven’t been linked to any deaths or serious injuries,” they went on to note that “the company’s product failures, all detected after the drugs had been sold to pharmacies and other distributors, suggest its internal quality assurance system is not working, medical experts consulted about the data told Bloomberg.”
DKT’s founding and funders
DKT International was founded in 1989 by Philip Harvey, who resigned as president in 2013 when he was replaced by Christopher H. Purdy, who serves as the organization’s current CEO. While Harvey passed away in 2021, he regularly funded DKT with money he earned from selling pornographic films and sex toys through his mail-order company, Adam & Eve.
DKT’s donor list boasts abortion philanthropists like the Buffett, Packard, Hewlett, and Gates Foundations. In 2016 alone, the Bill & Melinda Gates Foundation awarded DKT International a combination $25 million grant and $12.5 million loan to “expand the sale of contraceptives.” According to Inside Philanthropy, “The Gates Foundation has been funding DKT’s sexual and reproductive health work since the late 1990s, starting with a $4.3 million grant… In 2001, Gates began supporting DKT’s work specifically related to birth control and family planning.”
The Gates Foundation’s grants to DKT International for “family planning” have been in the millions, totaling nearly $90 million since 1999. According to public reports, between 2014 and 2019 the David and Lucile Packard Foundation contributed nearly $3.5 million in grants to DKT, Hewlett contributed $4.4 million, while DKT International’s largest donor, the Buffett Foundation, granted over $170 million (between 2013 and 2020) to the international abortion pill company.
DKT’s board currently includes Matthew Reeves, M.D, an abortionist founder of the Dupont Clinic in D.C., which advertises late abortion after 26 weeks of pregnancy and recently announced plans to expand as an “all-trimester” facility in California. The opening of that facility in Beverly Hills appears to have been thwarted due to pro-life intervention. Reeves’ online bio reveals he served as Medical Director at the National Abortion Federation. His LinkedIn page shows that since 2018, he has served as Chairperson of the National Medical Committee of the Planned Parenthood Federation and worked at Planned Parenthood of Metropolitan D.C. and the now-disaffiliated Planned Parenthood Golden Gate.
DKT’s U.S. abortion pill division
Bloomberg’s investigation failed to note is that DKT International’s U.S. division (FemHealth USA, Inc.), operates under the name Carafem as a national abortion pill chain. FemHealth’s connection is confirmed by DKT’s financial audits with Carafem’s donation page, clearly writing that FemHealth USA is “doing business as carafem.” And according to an archived page from Carafem’s website, FemHealth was “established in 2013 as a 501c3 non-profit social enterprise to deliver early, safe abortion services and family planning products in the USA and to take advantage of new efficiencies, new technology, and new approaches in the delivery of care.”
Carafem, founded in 2013, was part of Gynuity Health Project’s infamous telabortion trials, which led to the Food and Drug Administration’s (FDA) decision to permanently allow abortion pill sales by mail. DKT CEO Christopher Purdy also serves as Carafem’s President and CEO.
Carafem’s latest (2023) annual report boasts DKT International as a major donor. Both Carafem (the public face of FemHealth) and DKT International have deep connections to some of the richest billionaire abortion philanthropists in the world and are funded by the Packard Foundation, which invested millions into the U.S. abortion pill manufacturer Danco as well as the generic manufacturer, GenBioPro.

DKT International US Division (FemHealth USA) is abortion pill chain Carafem
Purdy denied any quality control issues with DKT, telling Bloomberg: “To suggest… that DKT accepts low quality, has somehow covered up less than acceptable quality misoprostol, or is motivated by money to allow low quality products to be sold, is incompatible” with the facts. Purdy also claimed that DKT had not received any complaints about Synokem’s products.
Synokem abortion pills enter U.S.
“Some DKT abortion medication kits made by Synokem, part-owned by Boston-based private equity firm TA Associates, are now entering the US, even though they haven’t been approved by the Food and Drug Administration. The kits, which contain misoprostol and mifepristone, are being purchased through underground online pharmacies that have no affiliation with DKT…” Bloomberg.com claimed.
One unregulated company is likely Aid Access, which also ships abortion drugs into the U.S. without FDA approval. Aid Access recently claimed the organization had “revamped its operation to let doctors in Democratic-led states with ‘shield laws’ … mail abortion pills to states where the medication is banned.”
In March of 2019, the FDA sent a warning letter to AidAccess.org to cease shipments of the pills to the U.S. A lawsuit filed in the case suggested the Aid Access abortion combipack shipped to the U.S. (product labeled “A-Kare”) was manufactured by Synokem and marketed to Aid Access by DKT International. The FDA letter, referred to in the lawsuit as “exhibit B,” can be seen below:

FDA letter to Aid Access notes abortion pill a-Kare marketed by DKT India
A study conducted between 2020 and 2021 by Concept Foundation and the International Planned Parenthood Federation (IPPF), shared with Bloomberg, concluded that “a significant problem still exists in relation to the quality of medical abortion drugs in low- and middle-income countries.”
The authors also found that “Misoprostol products in several geographically diverse, large countries have significant quality issues.” In addition, the study, “Quality testing of mifepristone and misoprostol in 11 countries,” found evidence of “mifepristone quality issues with 23.7% of samples tested non-compliant.”
Bloomberg also said the study “found that eight Synokem samples it analyzed contained levels of impurities in excess of international standards. Those samples were purchased in several markets where Synokem’s products are sold, including India, the Democratic Republic of Congo, Nigeria, Cambodia and Uganda….”
“Two of those were A-Kare, the DKT-branded product that US consumers can buy through unregulated online sources that aren’t affiliated with the organization. The rest were other products sold by Synokem directly,” Bloomberg alleged, adding, “Synokem makes drugs for India’s domestic market and subcontracts for big generic pharmaceutical companies such as Sun Pharmaceutical Industries Ltd., Cipla Ltd. and Macleods Pharmaceuticals Ltd., according to its website.”
This is potentially problematic; Live Action News previously located an import report which appears to show that at one time the U.S. generic abortion pill manufacturer, GenBioPro, received mifepristone tablets from Sun Pharmaceutical Industries, Ltd.
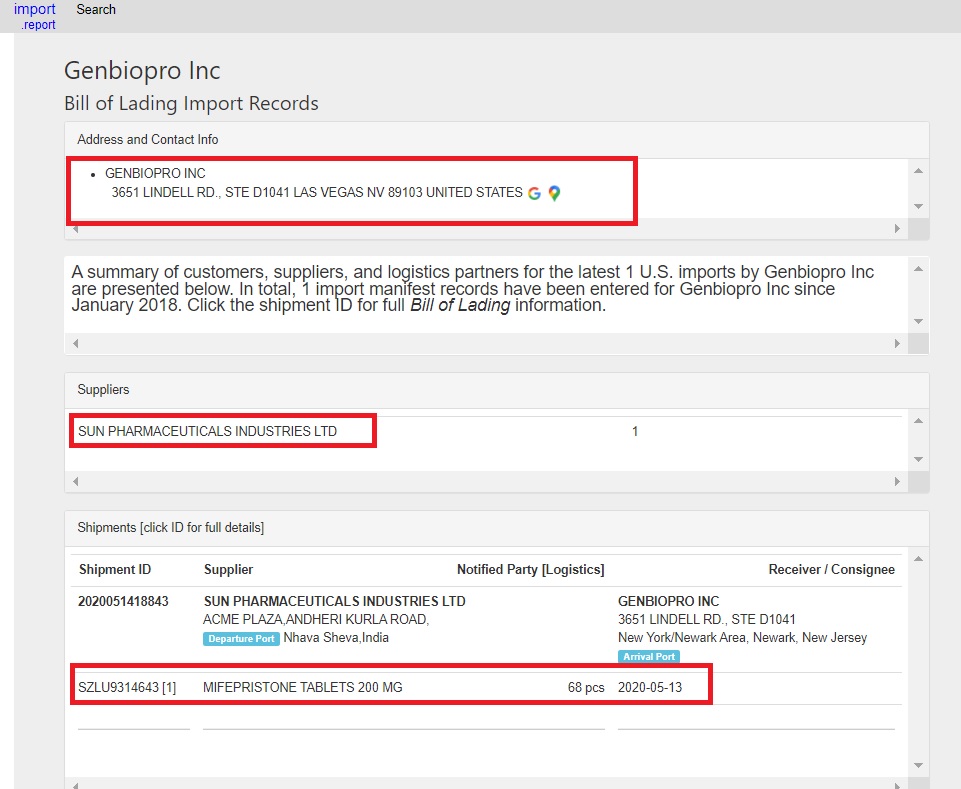
GenBioPro FDA Import Report abortion pills by Sun Pharmaceuticals
DKT encouraged to take quality seriously
“Three public health experts told Bloomberg that they have called on DKT employees or the organization’s donors to encourage it to take quality more seriously and to work with manufacturers that meet international standards. One said they spoke directly with the Gates Foundation and asked it to intervene but got no meaningful response. They all asked that their names not be used because they’re still working in the industry,” Bloomberg claimed.
The outlet added, “DKT hasn’t adopted some common safeguards to ensure medicine quality. The organization does not conduct inspections of Synokem’s facilities, according to Craig Darden, director of the nonprofit’s Mumbai-based program. Such inspections can help ensure good manufacturing practices are being followed. Instead, DKT said it relies on regular testing of the company’s products by an independent lab.”
DKT’s very lucrative business
DKT’ International’s total revenue in 2021 was $270.2 million, according to its most recent annual report. DKT International generated 6.1 million abortion pill combipacks in 2022, a 74% increase from the 3.5 million mifepristone/misoprostol combipacks they reported in 2018.
“Unlike a typical nonprofit that relies primarily on donations, DKT depends on sales… contributing to large bonuses earned by its executives,” Bloomberg wrote.
DKT CEO Christopher Purdy, whose compensation of almost $1 million included a $250K bonus in 2021, denied the insinuation that profit trumps safety. “We have no concerns that DKT’s bonus structure would, in any way, allow for a compromise on product quality,” Purdy told Bloomberg. “Quality is the number one criterion for choosing our product manufacturing,” he said.
Still, Bloomberg noted that the harms of potentially poor-quality abortion pills like mifepristone can reduce their efficacy and result in incomplete abortions. And, in the case of misoprostol, Bloomberg warned that “taking a product that fails to stop bleeding” after childbirth can be fatal.








