A Freedom of Information Act (FOIA) lawsuit recently filed by Judicial Watch (JW) is seeking abortion pill stability and manufacturing information from the U.S. Department of Health and Human Services (HHS), which oversees the U.S. Food and Drug Administration (FDA).
Tom Fitton, president of the watchdog organization, said the group was forced to sue after the FDA failed to respond to three FOIA requests. He called it “outrageous” that Judicial Watch had to sue in federal court “for basic safety information about the abortion pill, which is being pushed on women by a desperate pro-abortion movement.”
“Our experience is that this chemical abortion pill did not and will not receive appropriate review from the politicized FDA,” Fitton also stated.

Judicial Watch sues HHS FDA over stability of abortion pill
According to Judicial Watch’s press release, they are seeking “records regarding drug stability test results, new drug applications and related materials of the abortion drug Mifeprex (Mifepristone, formerly known as RU-486), as well as requests for reviews and assessments of the manufacturing facilities DANCO and GenBio where the abortion pills are produced.” These should include records reflecting long-term stability, as well as accelerated and forced degradation results.
The abortion pill (Mifeprex or mifepristone) was approved in 2000 during what many described as a highly secretive and politicized process within the FDA. The pill’s manufacturer, Danco Laboratories, LLC, was set up as a sub-licensee of the Population Council early on while the FDA only approved GenBioPro’s abbreviated new drug application for a generic Mifeprex on April 11, 2019.
Stability testing
Stability testing verifies that the drug can maintain its effectiveness under a specified storage condition as well as during the proposed shelf-life of the drug. This is important, given the fact that distribution of the abortion pill via mail-order delivery is expanding, while in-person distribution decreases.
JW’s lawsuit requested:
- Stability test results for the drug Mifeprex and its generic equivalent, mifepristone, pursuant to ICH requirements, including but not limited to records reflecting long-term stability, and accelerated and forced degradation results.
- Drug Product Transportation Stability studies for the drug Mifeprex and its generic equivalent, mifepristone, including but not limited to the expiry and temperature statements.
- Dissolution test results for the drug Mifeprex and its generic equivalent, mifepristone.
FDA’s website states, “FDA regulations require drug applicants to provide stability testing data with a proposed expiration date and storage conditions when they submit an application for FDA approval of their drug. This testing is designed to provide confidence that the product will meet the applicable standards of strength, quality, and purity throughout its shelf-life. The FDA verifies that an applicant’s proposed expiration date is supported by appropriate studies that the applicant has conducted.” It goes on to state that the “FDA recommends that applicants and manufacturers follow the recommendations in internationally harmonized guidance documents on stability testing such as the International Council on Harmonization guidance documents (ICH) Q1(A-F) and Q5C.”
GenBioPro’s website (as well as the pill’s label) states that the abortion pill must be stored “at 25°C (77°F); exclusions permitted to 15° to 30°C (59° to 86°F).” It adds that the pill should be protected from light. So, it seems reasonable to review abortion pill stability testing now that mail-ordered abortion pills could potentially be left for hours inside mailboxes at temperatures either above or below storage requirements.

Abortion Pill Storage Information GenBioPro website accessed 11082022
Abortion pill shelf life
The FDA’s website states, “Drug expiration dates reflect the time period during which the product is known to remain stable, which means it retains its strength, quality, and purity when it is stored according to its labeled storage conditions.” The FDA website notes, “there are several potential harms that may occur from taking an expired medicine or one that may have degraded because it was not stored according to the labeled conditions.”
According to ordering information published by Reproductive Access, generic mifepristone has a three-year expiration date. Mifeprex® has a five-year expiration date. Abortionist Daniel Grossman made a similar claim on Twitter (without documentation), noting that the second of the two-pill regimen, misoprostol, is good for two years. Yet, as recently as August of 2019, Reproductive Access claimed mifepristone only had a shelf life of “18 months.”
Shelf life is important now that abortion websites are dispensing abortion pills in advance of a pregnancy, and against the FDA’s guidelines. According to Politico, “Some telemedicine providers, including Choix, as well as in-person providers, have begun offering the pills before pregnancy as a way to expand access to abortion after the Supreme Court’s June decision that gave states the right to ban the procedure.”
Politico also reported that an FDA spokesperson told them that if the abortion pill were prescribed “before a patient is pregnant, providers wouldn’t be able to properly oversee care to ensure safety and effectiveness.” But the media outlet added that although advanced provision of abortion pills is clearly against FDA regulations, “[t]he spokesperson also did not say if the agency has taken any enforcement actions for misprescribing of mifepristone, but the FDA can enforce its regulations by fining violators, seizing drugs or imposing an injunction.”
Abortion pill manufacturing location and inspections
The Judicial Watch lawsuit is also seeking “[r]eports from all FDA inspections and assessments of DANCO and GenBio manufacturing facilities, and assessment of compliance with applicable laws and regulations.”
It is important to note that, from the beginning, the abortion pill’s manufacturer was kept secret, as were the company’s principals and investors — so secret that many media outlets even questioned it. The location of manufacturing plants remains elusive even today, despite the abortion pill being used over five million times and sold to minors.
In 2000, China officials confirmed the Shanghai-based Hua Lian Pharmaceutical Co. (owned by the Chinese Communist Party) was manufacturing the raw compound for the abortion pill. Reports from 2008 identified China-based Shanghai Hualian (tied to tainted leukemia drugs) as the sole supplier of mifepristone to the U.S.
According to Fox News, “The…manufacturing site switched from China to Europe” earlier this year. Live Action News previously located a bill of lading import record involving the generic pill which showed abortion drugs were shipped from Sun Pharmaceuticals’ Mumbai location after departing from a port in Nhava Sheva, India.
FDA told Live Action News they would not disclose the abortion pill manufacturing site, writing “Consistent with our obligations under applicable laws, including the Trade Secrets Act, the FDA does not disclose information submitted by the Applicants to FDA about manufacturing sites for Mifeprex and the generic for Mifeprex, Mifepristone Tablets, 200 mg.”
Live Action News also asked the FDA whether they inspect manufacturing facilities involved with the abortion pill, and they responded in writing: “The FDA conducts inspections and assessments of FDA regulated drug manufacturing facilities to determine a firm’s compliance with applicable laws and regulations.”
But while an archived page on the FDA website reveals that, in 2010, the FDA inspected Danco Laboratories, additional inspection data is difficult to locate.
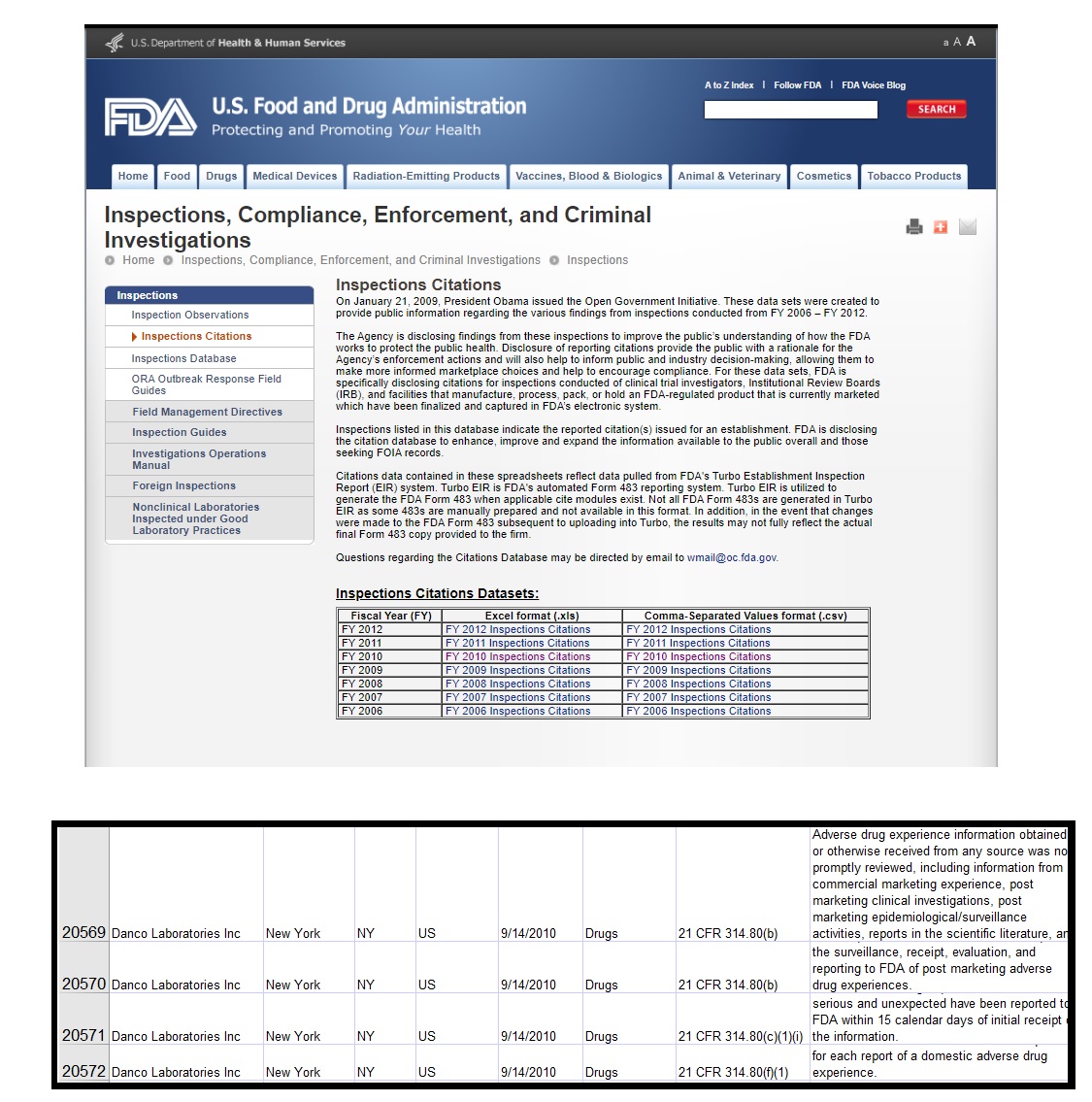
Inspections of abortion pill manufacturer Danco in archived FDA website
“The Biden administration wants the pill to have wide access, so anything that threatens that agenda will be kept secret,” Fitton told Fox News Digital. “If this pill is as important as the Biden administration suggests, then American women have the right to as much information as possible on its safety and efficacy.”
In addition to attorneys’ fees and other litigation costs, the lawsuit is asking that the court require HHS to produce “any and all nonexempt records” and enjoin them from continuing to withhold such records.








