Just days after 100 lawmakers sent a letter to the Food and Drug Administration (FDA) — which oversees drug safety — requesting that the FDA monitor abortion group Aid Access for illegally dispensing abortion pills, Aid Access announced its intent to defy the FDA’s warnings to “immediately cease” dispension of the drugs.
Aid Access founder Rebecca Gomperts published her response on Aid Access’s website as well as on Women on Waves, another organization Gomperts founded in 1999. Gomperts claims, “Last year, I prescribed 2,581 medical abortions out of 11,108 women who consulted me.”
Gomperts states in part:
On March 8, 2019, I received a letter from the FDA ordering my new (since 2018) organization, Aid Access, to stop providing telemedical abortion services to women who cannot otherwise access safe abortions because of costs, domestic violence, distance, or other reasons, and who do not have access to other doctors willing or able to prescribe misoprostol and mifepristone.
This letter was applauded by Republican members of Congress, of whom 92 percent are male.
But I will not be deterred. When U.S. women seeking to terminate their pregnancies prior to 9 weeks consult me, I will not turn them away. I will continue to protect the human and constitutional right of my patients to access safe abortion services.
Notably, the Congressional letter was signed by a number of women in Congress, including Reps. Martha Roby, Carol D. Miller, Virginia Foxx, Liz Cheney, Ann Wagner, Jenniffer González Colón, Debbie Lesko, and several medical doctors who also serve in the House of Representatives, among many others.
Rebecca Gomperts founder of Aid Access defiant to offer illegal abortion pills online (Image: Twitter)
In a statement to Live Action News, the FDA wrote:
As noted in the FDA warning letter to AidAccess issued on March 8, 2019, failure to correct the violations of the law may result in FDA regulatory action, including seizure or injunction, without further notice. We cannot comment on a potential future action at this time, but we remain very concerned about the sale of unapproved mifepristone for medical termination of early pregnancy on the Internet, because this bypasses important safeguards designed to protect women’s health. Unapproved drugs purchased from foreign internet sources are not the FDA-approved versions of the drugs, and therefore, they are not subject to FDA-regulated manufacturing controls or FDA inspection of manufacturing facilities. Drugs that have circumvented regulatory safeguards may be contaminated, counterfeit, contain varying amounts of active ingredients, or contain different ingredients altogether.
The statement went on to say that “The FDA’s regulation and oversight of the drug approval process and drug distribution helps to protect patients by applying rigorous scientific and safety standards, requiring labeling review for accuracy and completeness, and working to ensure that counterfeit and unsafe medicines do not enter the U.S. drug supply. When these requirements are not met, FDA can step in to help protect consumers.” The FDA reiterated that it has warned AidAccess.org and Rablon that they are in violation of the Federal Food, Drug, and Cosmetic Act for causing the introduction of unapproved new drugs into U.S. commerce.
Drugs are tightly regulated by the FDA for a reason, and under current requirements, the abortion pill — also known as Mifeprex or Mifepristone — is only permitted to be dispensed in hospitals or clinics by medical personal who are approved prescribers of the pill. FDA placed Mifeprex under a tighter regulatory system known by the acronym REMS (Risk Evaluation and Mitigation Strategy), “a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use.”
Under the Mifepristone REMS Program, the FDA states, “Mifeprex and the approved generic version of Mifeprex” may:
- [O]nly be supplied directly to healthcare providers who are certified to prescribe the drug product and who meet certain qualifications.
- [T]he products are only available to be dispensed in certain healthcare settings, specifically, clinics, medical offices and hospitals, by or under the supervision of a certified prescriber.
- They are not available in retail pharmacies and are not legally available over the Internet.
The FDA has warned women against purchasing these dangerous pills online: “Do Not Buy Mifeprex Over the Internet.”

FDA warns consumers to not buy abortion pills over the internet (Image: FDA)
The FDA adds, “You should not buy Mifeprex over the Internet because you will bypass important safeguards designed to protect your health (and the health of others). Mifeprex has special safety restrictions on how it is distributed to the public. Also, drugs purchased from foreign Internet sources are not the FDA-approved versions of the drugs, and they are not subject to FDA-regulated manufacturing controls or FDA inspection of manufacturing facilities.”
READ: AWFUL: Abortion groups tell women to lie about abortion pill, claim miscarriage
Weeks ago, the FDA updated its adverse events report through 2018, documenting “24 deaths of women associated with Mifeprex since the product was approved in September 2000….” The FDA also noted at least 4,200 additional adverse effects. Under 2016 changes, the drug’s manufacturer, Danco, no longer has to report non-fatal adverse effects, so we can only imagine what the number really is.
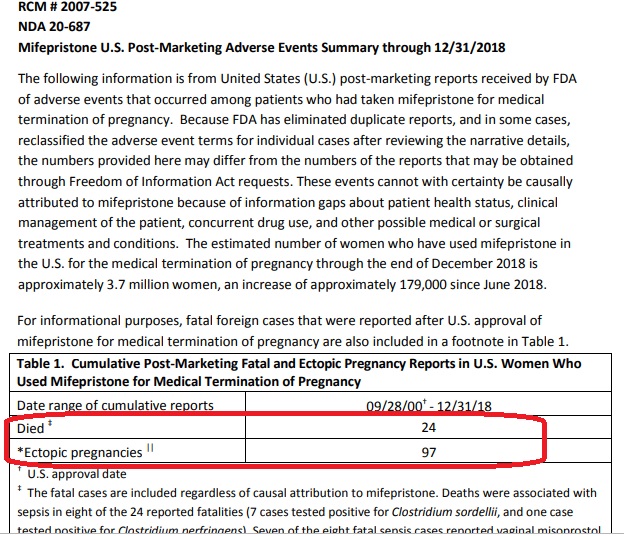
FDA reported deaths for abortion pill December 2018
Live Action News previously documented a larger push by pro-abortion organizations to lift the FDA’s REMS requirements, to expand abortion pill dispension to mail order and online sales, via self-managed abortions. And we also documented the secretive history of Danco’s investors, which include organizations such as the Packard Foundation and other abortion philanthropists, who appear to stand to gain financially from a broad abortion pill expansion. The original drug, Mifeprex, was approved in 2000 after being brought to the U.S. by the eugenics-founded Population Council.
The defiance by Aid Access comes on the heels of multiple pro-life bills being passed in several states. Pro-abortion organizations, who used to claim that self-abortions injure and even kill women, now appear willing to place them in harm’s way. In addition, when these same women experience serious complications from these dangerous pills, they are instructed to present to the ER and lie, claiming they had a miscarriage. Ironically, while the abortion lobby shows support to Aid Access, they simultaneously want to discredit the science behind abortion pill reversal.
Editor’s Note 5/17/19: This article was edited to include a statement from the FDA.
Editor’s Note 4/18/21: The FDA has received reports of serious adverse events in women who took Mifeprex. As of December 31, 2018, there were reports of 24 deaths of women associated with Mifeprex since the product was approved in September 2000, including two cases of ectopic pregnancy resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal.
The adverse events cannot with certainty be causally attributed to mifepristone because of concurrent use of other drugs, other medical or surgical treatments, co-existing medical conditions, and information gaps about patient health status and clinical management of the patient. A summary report of adverse events that reflects data through December 31, 2018 is here.
Editor’s Note: FDA has received reports of serious adverse events in women who took mifepristone. As of June 30, 2021, there were reports of 26 deaths of women associated with mifepristone since the product was approved in September 2000, including two cases of ectopic pregnancy (a pregnancy located outside the womb, such as in the fallopian tubes) resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal. The adverse events cannot with certainty be causally attributed to mifepristone because of concurrent use of other drugs, other medical or surgical treatments, co-existing medical conditions, and information gaps about patient health status and clinical management of the patient. A summary report of adverse events that reflects data through June 30, 2021 is here.
“Like” Live Action News on Facebook for more pro-life news and commentary!