In keeping with an April decision not to enforce safety guidelines surrounding the abortion pill, the Biden administration has announced its intention to revisit the FDA guidelines that have been in place since 2000.
The FDA announced on May 7, that it would undertake a review of the current FDA Risk Evaluation and Mitigation Strategy (REMS) safety protocols in place for the abortion pill, also known as mifepristone. As Live Action has reported, abortion activist groups had been pressuring the Biden administration to remove the regulations under the premise that “underserved” populations would benefit from easier access. Based on the stay filed by the Biden administration, the review will likely conclude before December 1, 2021, as reported by the National Catholic Register. The Biden administration’s latest policy shift comes just one month after the FDA loosened restrictions to allow the abortion pill to be distributed by mail for the duration of the COVID-19 pandemic.
The REMS protocol stipulates that the abortion pill must be dispensed by a certified prescriber and administered in a medical setting. This helps to ensure a patient has a doctor following her care, including ensuring she is not experiencing an ectopic pregnancy, in which case the abortion pill could cause hemorrhaging and death. The protocol also prevents the abortion pill from being administered too far along in the pregnancy and helps to prevent the pill from being sold online. The abortion pill has been found to be four times more dangerous for women than first-trimester surgical abortion. The second part of the abortion pill regimen involves taking misoprostol, which forces the patient to expel her deceased, preborn baby at home.
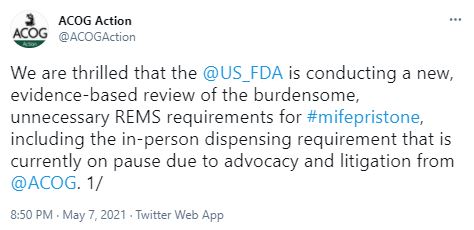
The pro-abortion American College of Obstetrics and Gynecologists (ACOG) praised the FDA’s decision on Twitter: “We are thrilled that the @US_FDA is conducting a new, evidence-based review of the burdensome, unnecessary REMS requirements for #mifepristone, including the in-person dispensing requirement that is currently on pause due to advocacy and litigation from @ACOG.”
Pro-abortion groups like ACOG have decided that the “good” of abortion trumps any risk to patients and have recently updated their recommendations with this premise in mind, ignoring that abortion complications are underreported. As noted in a U.S.-based 2021 study, “the surgical management of over half the complications was performed by someone other than the abortion provider, yet treating physicians are not required to report complications.” A recent report from Right to Life U.K. has also shown that abortion pill complication rates are five times higher than reported in official statistics, and that an average of 250 women per month required a surgical procedure after taking the abortion pill.
Additionally, as Live Action founder and president Lila Rose noted, the absence of REMS guidelines will further erode reporting requirements. “In reality, there is no requirement for the manufacturer of the pill, Danco Laboratories, or its generic, GenBioPro, to report any adverse reactions except death under REMS since 2016. In addition, there are no federal laws in place to require or track abortion complications across all states.”
Planned Parenthood president Alexis McGill Johnson praised the FDA’s review in a statement posted on its website: “This is an important step in the right direction to expand access to reproductive health care and achieve health equity. We appreciate FDA leaders for reviewing the science and taking these initial steps. We have long known that the decades-old restrictions on mifepristone are medically unnecessary.”
READ: Pro-abortion leader in Argentina dies after legally taking drug to induce abortion
Though the relaxing of the FDA rules has proven beneficial for the abortion industry, the rules are very necessary to protect women. Shortly after the United Kingdom approved at-home abortions during the COVID-19 pandemic, a 28-week preborn baby died after his or her mother took abortion pills beyond the allowed 10-week limit, and two women died as well. Nearly four million preborn children have been killed by the abortion pill to date, and at least 24 women have died in the U.S.*
*Editor’s Note: The FDA has received reports of serious adverse events in women who took Mifeprex. As of December 31, 2018, there were reports of 24 deaths of women associated with Mifeprex since the product was approved in September 2000, including two cases of ectopic pregnancy resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal.
The adverse events cannot with certainty be causally attributed to mifepristone because of concurrent use of other drugs, other medical or surgical treatments, co-existing medical conditions, and information gaps about patient health status and clinical management of the patient. A summary report of adverse events that reflects data through December 31, 2018 is here.
“Like” Live Action News on Facebook for more pro-life news and commentary!