An abortion pill lawsuit set to be heard before the United States Supreme Court on March 26, 2024, will not result in the removal of the drug from the market, as some have suggested. Here’s what you need to know…
FACT: Abortion drugs have been available since approval in 2000 and will continue to be available after the case is heard
The case, Alliance for Hippocratic Medicine (AHM) v. Food and Drug Administration (FDA), challenges the FDA’s more recent expansion of the drug mifepristone (Mifeprex). AHM Plaintiffs are represented by Alliance Defending Freedom (ADF).
The FDA’s more recent expansions of the drug occurred when the FDA moved away from the required in-person visits to obtain the pills (where medical professionals would likely provide more comprehensive medical assessments or examinations prior to prescribing the pills), to approving the dispensing of abortion drugs virtually, by mail, or at the pharmacy counter — where little to no time would be spent evaluating the client.
In AHM’s abortion pill lawsuit, “The Court is reviewing the FDA’s recklessness in removing nearly all its original safety standards for the use of abortion drugs to the detriment of women’s health and safety. Regardless of the outcome, abortion drugs will remain available in the United States. We are simply asking the Court to reinstate the original standards that were in effect for over 15 years,” Alliance Defending Freedom Senior Counsel Erik Baptist told Live Action News.
The abortion industry wants you to believe certain narratives about our upcoming Supreme Court case against the FDA.
It's time to separate myth from fact. Let's dive in 🧵
— Alliance Defending Freedom (@ADFLegal) March 20, 2024
Lawsuit Background
The lawsuit, Alliance for Hippocratic Medicine (AHM) v. Food and Drug Administration (FDA), was initiated in November of 2022 by a group of medical doctors and organizations who claimed the 2000 approval of the drug was illegal and the expanded use of the abortion pill was unsafe. The doctors testified to treating women with severe complications — “many who presented to the emergency room.”
On April 7, 2023, U.S. District Court Judge Matthew J. Kacsmaryk ruled in favor of the plaintiffs to immediately suspend the 2000 approval and subsequent FDA changes to the abortion pill. But the case was appealed to the U.S. Court of Appeals for the Fifth Circuit, which issued a partial stay.
In May of 2023, AHM’s abortion pill lawsuit went back to be heard again by the Fifth Circuit where a 62-page decision issued August 16, 2023, by the Appeals Court ruled to allow mifepristone to remain “available to the public under the conditions for use that existed” prior to 2016. That decision would therefore remove mail-order and pharmacy dispensing of the drug while leaving in place the FDA’s 2000 approval as well as its 2019 approval of the generic version (GenBioPro).
The pill has remained available under an April order from the U.S. Supreme Court and will stay in effect until the high court hears the case later this month.
FACT: The FDA Removed Essential Safeguards for the Abortion Pill
AHM’s brief claimed that the “FDA’s removal of safeguards for abortion drugs was arbitrary, capricious, an abuse of discretion, and otherwise unlawful.”
Alliance Defending Freedom, which is representing AHM, claimed that its clients are “on the front lines witnessing the harms the FDA has caused” by caring for women and girls “facing severe health complications because of the FDA’s illegal actions.”
According to ADF, the FDA’s 2000 approval identified as essential that:
- Women must be seven weeks pregnant or less to take the drugs;
- Women must have at least three in-person doctor visits to prevent severe and even life-threatening complications;
- A doctor must prescribe the drugs; and
- A doctor must report all complications women suffer from the drugs.
AHM’s abortion pill lawsuit is asking the Supreme Court to reinstate “essential safeguards” put in place on the abortion pill in a safety system called REMS by the FDA prior to 2016, when the FDA chose to “eliminate the requirement that prescribers report all serious adverse events,” AHM claimed in its brief.
AHM added that “for 2016, FDA failed to consider a major aspect of the problem: ‘the cumulative effect’ of the interrelated changes.” The FDA’s changes, according to AHM, “increased the risk that more women taking abortion drugs will need emergency care” in three ways:
- The FDA “increased the gestational-age limit from seven to ten weeks” where the “‘failure rate’ climbs from roughly 2 to 7 percent, as confirmed by FDA’s label.”
- The “FDA’s removal of the Day 14 in-person follow-up visit” will “naturally result[] in more women report[ing] to the emergency room.’”
- The FDA’s decision to end the “requirement that licensed doctors prescribe and provide ongoing care to women using abortion drug” results — as the FDA concedes — in “OB/GYNs, OB/GYN hospitalists, and emergency room doctors like Respondents ‘who must manage the aftermath.’”
ADF emphasized that it is the FDA’s job to keep people safe, but claimed that the FDA abandoned that responsibility when it recklessly removed its own safety standards for abortion drugs.
“After requiring critical safety standards for 16 years, the FDA removed them without sufficiently evaluating the impact on women’s health. That failure violated the agency’s duty to women and girls,” the organization claimed.
FACT: Abortion Drug Label Suggests Potential Risks
AHM has claimed in its brief that abortion pill-related emergency room visits are estimated to be in the tens of thousands, based on published percentages for ER visits on the drug’s 2023 label which state that “2.9 to 4.6 percent of women who take abortion drugs end up in the emergency room.”
This, AHM claimed, suggests “roughly one in 25 women who take mifepristone will end up in the emergency room.”
MYTH 2: “This case could take abortion drugs off the market.”
TRUTH: SCOTUS is not considering the drug’s approval, which means it won’t be taken off the market. We’re asking for the restoration of basic safety standards the FDA illegally removed, like in-person doctor visits.
— Alliance Defending Freedom (@ADFLegal) March 20, 2024
In addition, AHM claimed that the FDA’s medication guide acknowledges that as many as seven percent (7%) of women will need surgery after taking mifepristone ‘to stop bleeding’ or to complete the abortion.
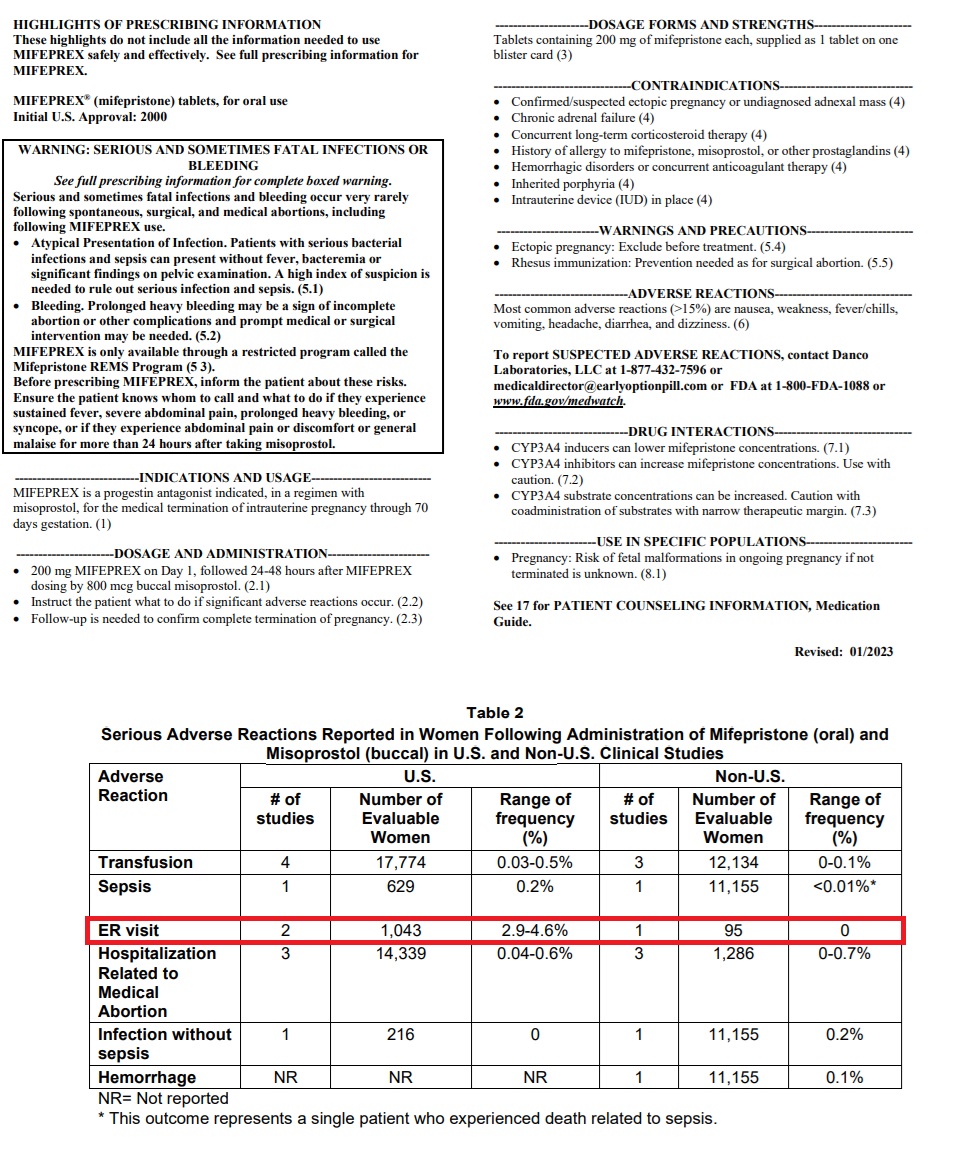
Mifepristone Jan 2023 label shows percentage of women taking abortion pill visit ER
“FDA’s current label for mifepristone continues to require a Black Box warning because the drug can cause ‘[s]erious and sometimes fatal infections and bleeding’… It also directs women to emergency rooms if one of many adverse complications arise,” AHM wrote in the brief.

Abortion pill Black Box warning and medication guide suggests sending women to ER
FACT: Abortion Pill Documents Suggest Tens of Thousands Could Seek Emergency Care
Data for 2023 indicates that abortion pill use increased nearly 31% (30.57%) from the 492,210 reported abortion in 2020 (53% of all abortions) to 642,700 abortion pills recorded by 2023 (63% of all abortions).
- Estimating ER visits from the newly published abortion pill data for 2023 reveals that out of the 642,700 abortion pills recorded by Guttmacher, between 19,000 (18,638) and 30,000 (29,564) women who took abortion drugs may have potentially ended up in the emergency room in 2023.
- In addition, using the 2-7% figure off the medication guide, we estimate that between 13,000 and 45,000 women who took the abortion pill in 2023 potentially required “a surgical procedure because the pregnancy did not completely pass from the uterus or to stop bleeding.”
These percentages, backed up by other studies, are not small numbers, which is why AHM is asking that SCOTUS turn back the safety requirements to pre-2016. By doing so, women may have more thorough care in clinic than they are likely receiving now. Today, abortion insiders have even suggested that women should lie and claim to be miscarrying naturally when they present to the emergency room with abortion pill complications. This dampens any chance that accurate numbers on abortion pill complications or deaths will be reported as such to authorities.
“SCOTUS is not considering the drug’s approval, which means it won’t be taken off the market. We’re asking for the restoration of basic safety standards the FDA illegally removed, like in-person doctor visits,” ADF wrote recently on X, adding, “EVERYONE who supports government accountability & women’s safety should support this case. Women deserve an FDA that watches out for THEIR health—not the health of the abortion industry.”







