The case Alliance for Hippocratic Medicine (AHM) v. Food and Drug Administration (FDA), regarding the status of the abortion pill, was argued Wednesday before the United States Court of Appeals for the Fifth Circuit. The lawsuit challenged the legitimacy of the drug’s 2000 approval — and at least one of the judges pushed back on the narrative that the FDA could ‘do no wrong,’ even suggesting the move to weaken the REMS for mifepristone and remove required reporting of non-fatal adverse events was not “very pro-science, pro-information, pro-intellectual curiosity” of the agency.
Tension arose when Circuit Court Judges Jennifer Walker Elrod, James Ho, and Cory Wilson grilled opposing counsel about disparaging remarks the abortion pill manufacturer (Danco Laboratories) made in its motion for a stay against the previous District Court’s ruling. That ruling was issued by U.S. District Court Judge Matthew J. Kacsmaryk, who suspended the FDA’s 2000 approval of the abortion pill mifepristone and all FDA abortion pill decisions made thereafter. A temporary stay was later issued by the Supreme Court until the case could be heard on appeal.
Arguing on behalf of the plaintiffs, Alliance Defending Freedom (ADF) attorney Erin Hawley told the Court, “This case isn’t about ending abortion, it’s about challenging a particularly dangerous type of abortion.”
Danco Called Out for ‘Unusual Remarks’
Opposing counsel Jessica Lynn Ellsworth (representing abortion pill manufacturer Danco Laboratories) and Deputy Assistant Attorney General Sarah Harrington (representing the FDA) were argumentative at times, prompting the Circuit Judges to lecture the pro-abortion attorneys.
In one instance, Judge Elrod challenged Danco’s legal counsel, telling her, “I am concerned about some rather unusual remarks in the filings.” Judge Elrod was speaking about Danco’s criticism of the District Court.
She suggested that the wording used in Danco’s brief disrespected the District Court. The brief claimed the Court defied “long standing precedent” and labeled the injunction “an unprecedented judicial assault.” It also referred to the court’s “relentless one-sided narrative,” even describing it as a “non-expert court,” and adding that the Court exhibited “equally groundless bending every settled rule.”
The circuit judge said the remarks were ones “we normally don’t see from learned counsel.”
When Danco’s lawyer refused to back down, Judge Elrod jumped back in, asking, “So you think it was appropriate to attack the district court personally in the case in that way?”
Ellsworth then reluctantly conceded, “With more time, we may have ratcheted down some of that.”
Is Pregnancy an ‘Illness?’
Tension could also be heard during discussion over whether pregnancy was an “illness,” a classification allegedly used to “fast-track approval authority” of the drug — according to plaintiffs.
“The only way the FDA could have approved chemical abortion drugs was to use its accelerated drug approval authority, necessitating the FDA to call pregnancy an ‘illness’ and argue that these dangerous drugs provide a ‘meaningful therapeutic benefit’ over existing treatments,” pro-life plaintiffs wrote in their initial complaint.
“But pregnancy is not an illness, nor do chemical abortion drugs provide a therapeutic benefit over surgical abortion,” they added.
This point prompted Circuit Court Judge James Ho to proclaim to Danco lawyer Jessica Ellsworth that, “Pregnancy is not a serious illness.”
Judge Ho then asked opposing counsel, “When we celebrate Mother’s Day – are we celebrating illness?”
Debating the Issue of Standing
AHM’s lawsuit also alleged that the FDA’s lax requirements have essentially violated the conscience rights of pro-life emergency room physicians or OBGYNs who are treating abortion pill complications.
Opposing attorneys disputed the claim and in return they argued that AHM did not have standing to bring the case.“There must be a specific doctor with a specific injury from the regulation,” Ellsworth claimed, suggesting no link was evident with these plaintiffs.
The judges appeared to push back, citing multiple places in the plaintiff’s declarations where the pro-life doctors testified they had to complete abortions on women who presented for emergency situations after a chemical abortion.
In arguing on behalf of AHM, ADF attorney Erin Hawley told the Court that “There is no question there is a substantial risk of harm. Plaintiff-doctors have been forced to participate in and perform abortions.” Hawley even pointed to mifepristone’s drug label, which clearly tells women to present to the ER if experiencing complications.

Abortion Pill Mifepristone Black Box Warning on March 2023 label
On behalf of the FDA, attorney Harrington disputed that under FDA’s requirement, which allows for mail order abortion pills and no in-person visit with a health care provider, women will present to an ER.
“It’s extremely, statistically unlikely that any woman will need to go to the emergency room to seek care after taking mifepristone,” Harrington claimed, later alleging that this amounted to less than one percent of all women who take mifepristone.
But ADF attorney Erin Hawley pushed back during her argument, directing the Justices to the March 2023 mifepristone label which shows that between 2.9% and 4.6% of women will likely present to the ER.
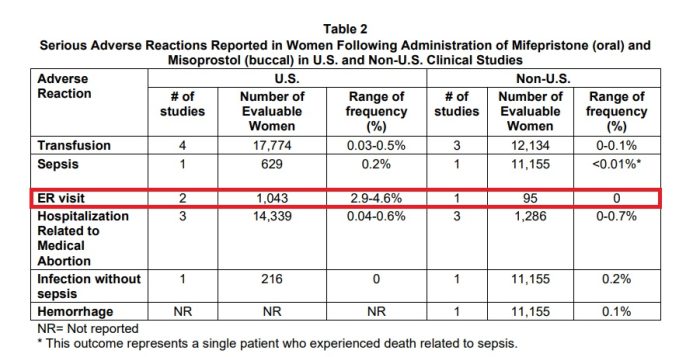
Abortion Pill Label March 2023 ER Visits Table 2
“Seeing and treating patients isn’t an injury,” the FDA’s attorney claimed.
On this matter Circuit Judge Cory Wilson weighed in:
It just strikes me that what the FDA has done in making this more available and doing it by mail order and removing the doctor visits as well as the requirement that the prescriber be a doctor, is you’ve made it much more likely that patients are going to go to emergency care or medical clinic where one of these [pro-life] doctors is a member. I don’t see how you square that circle.
Judge Reacts to ‘FDA Can Do No Wrong’ Theme
The Government’s counsel called the District Court’s decision which led to the appeal “unprecedented,” and claimed that the plaintiffs were “unlikely” to succeed in their claims.
The judges immediately responded and pointed out throughout the hearing that the FDA was not off limits to judicial scrutiny and even cited multiple examples where the FDA’s initial drug approval process for dispensing of a drug was called into question.
“I’m just wondering why not just focus on facts of the case rather than have this sort of ‘FDA can do no wrong’ theme?” Judge James Ho asked. “I don’t understand this theme,” he added, calling it the “narrative you all are putting forth.”
“Nobody should ever question the FDA – this is unprecedented?” Ho asked somewhat sarcastically. Judge Ho then brought up numerous examples where FDA decisions have been questioned or reversed.
He pointed to Makena, a “subpart-H drug for pregnant women” the approval of which was recently withdrawn by the FDA. Judge Ho even read the New York Times headline, “F.D.A. Rushed a Drug for Preterm Births. Did It Put Speed Over Science?”
Ho also pointed to criticisms of the FDA over unacceptable “food safety failures” and then claimed that the “FDA is being blamed for the opioid crisis.”
“What I am trying to say,” he said, sounding slightly irritated, “is it’s a theme you all are putting forth that is completely unnecessary. We’re allowed to look at the FDA just like we’re allowed to look at any agency. That’s the role of the courts.”

FDA rushed drug for preterm births NYTs
In response, Danco’s attorney suggested that courts should not “second guess the FDA.”
But, “FDA approved this drug [mifepristone] in 2000,” Judge Ho said, before proceeding to read an excerpt from the AMA Journal. “Of all the novel therapeutics approved by the FDA that decade, one-third of them have had safety issues,” Judge Ho claimed.
He then referred to amicus briefs filed in the case which cited “numerous problems with FDA approved drugs.”
“Do you know how many FDA drugs have been recalled?” Judge Ho asked Danco’s counsel.
“I do not know the answer to that question, your honor,” Ellsworth said in response.
Danco’s attorney Jessica Lynn Ellsworth then pointed to the FDA’s “adverse events reporting” as some sort of guardrail for catching potential concerns. Judge Ho reminded her, “But you all got rid of adverse events reporting [for the abortion pill] — the non-fatal ones,” he told her.
Judge Ho pressed in, asking Danco’s attorney why the FDA would remove the non-fatal adverse events reporting “right when you were expanding its use”.
“Why deprive the world of that information?” he asked, suggesting the move was not a “very pro-science, pro-information, pro-intellectual curiosity” position by the FDA.
In a press conference following arguments in the case, Hawley referred to the FDA’s “unprecedented and unlawful actions to approve chemical abortion drugs” and to “later remove those crucial safe guards” as political, claiming the FDA “placed politics ahead of women.”
“Now, we are asking the… Court to restore public safety and trust by demanding that the FDA follow proper science not politics… our women and our girls deserve better,” Hawley stated.
A decision in the case is expected within a few months.







