The United States Supreme Court finalized hearing oral arguments on March 26 in the Alliance for Hippocratic Medicine (AHM) v. Food and Drug Administration (FDA), a lawsuit which challenged the legitimacy of FDA’s recent expansions of the abortion pill, likely the most important case since the Dobbs v. Jackson case which overturned Roe v. Wade.
During oral arguments, Elizabeth Prelogar, Solicitor General representing the FDA, argued that respondents — a group of pro-life medical professionals — lacked standing to bring the lawsuit, claiming the medical professionals were promoting “judicial second guessing.”

US Supreme Court hears abortion pill case FDA v AHM March 26, 2024
Do pro-life doctors have standing?
AHM’s standing in the case was a predominant issue throughout the hearing. Yet, as Live Action News previously documented, the Fifth Circuit previously held that Respondent doctors and associations do have standing to file suit.
Another issue heavily argued was whether conscience protections applied to the Respondent pro-life doctors. This line of questioning seemed to intrigue several of the justices, many of whom inquired whether federal conscience protections would have sufficed, rather than — as Justice Ketanji Brown Jackson alluded — everyone being impacted by returning to pre-2016 FDA safety requirements for accessing the drug.
Those requirements would roll the gestational limits back from 10 weeks to seven weeks and eliminate mail-order and pharmacy dispensing of the drug by reinstating in-person requirements. It would also once again require prescribers of the drug to report non-fatal adverse events.
The FDA claimed that the Emergency Medical Treatment and Labor Act (EMTALA) would protect conscience rights — but Alliance Defending Freedom (ADF) attorney Erin Hawley, representing the pro-life doctors in the case, seemed to dispute that claim.
Hawley pointed to two specific clients, Dr. Christina Frances and Dr. Ingrid Skop, who testified to treating women in the emergency room who were suffering from incomplete abortions.
“These are emergency situations. Respondent doctors don’t necessarily know until they scrub into that operating room whether this will be abortion drug harm,” Hawley told Justice Ketanji Brown Jackson. “In addition, the government simply cannot get story straight on EMTALA… they told district court the very opposite.”

ADF attorney Erin Hawley represents pro-life doctors argues abortion pill case before SCOTUS
AHM’s arguments for standing
AHM doctors have testified to treating women who took abortion drugs with severe complications — “many who presented to the emergency room.”
“In removing crucial safeguards for the use of abortion drugs, FDA expressly counted on OB/GYN hospitalists and emergency-room doctors—like Respondents—to manage abortion-drug complications,” AHM previously claimed. “When faced with these emergencies, Respondents have no choice but to provide immediate treatment, even though this kind of participation in an elective abortion harms their consciences and injures them in other ways.”
They allege that the “FDA’s [own] data and the Doctors’ testimony establish that Respondents face a ‘substantial risk’ of these injuries… and FDA’s 2021 and 2016 actions increase that risk.”
The FDA has relied on unreliable and inadequate data to remove essential safety standards from #Mifepristone, but our patients deserve better care. pic.twitter.com/KLdGX9qo9S
— AAPLOG (@aaplog) March 26, 2024
According to AHM, while the FDA argues they do not have standing, the FDA relied on similar hospitals for clinical trials and published abortion pill studies. One of those was the University of California, a pro-abortion institution heavily tied to abortion pill investors.
SHOCK #1: FDA claimed to have “sovereign immunity” from lawsuits
“Is there anybody who could challenge in court the lawfulness of what the FDA did here?” Justice Samuel Alito asked the FDA’s attorney.
“In this particular case, I think the answer is no,” Prelogar stated.
Justice Alito reiterated, asking the FDA whether states can intervene, and again, Prelogar said “No, the states lack standing.”
“Okay, how about a doctor who opposes abortion. So, she’s on duty in an emergency room when a woman comes in with complication from having taken Mifepristone and… in order to save her life the doctor has to abort a viable fetus,” Alito asked. “Would that doctor have standing to seek injunctive relief?”
FDA responded by not “disputing” that it would violate conscience protections, and claimed the scenario was “unduly speculative.”
“Now, how about a woman who suffers adverse consequences from taking mifepristone. Would she be able to sue for damages?” Justice Alito asked.
“I expect that we would have sovereign immunity arguments in that case,” FDA asked.
“Is there anybody who can sue… shouldn’t somebody be able to challenge that in court? Who, in your view?” Alito asked.
The FDA again said no. “I think that with respect to these regulatory changes, it’s hard to identify anybody that would have standing to sue,” Prelogar told the Court.
“So, your argument is that it doesn’t matter if FDA flagrantly violated the law, didn’t do what it should have done, endangered the health of women, it’s just too bad – nobody can sue them in court?” Alito asked. “There’s no remedy, the American people have no remedy for that?”
In response, the FDA invoked article 111 and reiterated that the respondents had no standing.
SHOCK #2: FDA portrays tens of thousands of abortion pill ER visits as small
Arguments by the FDA suggested that only a small number of women will present to emergency rooms after taking the abortion pill, but in her arguments before the Supreme Court, ADF attorney Erin Hawley repeatedly cited published percentages for ER visits on the drug’s 2023 label which state that “2.9 to 4.6 percent of women who take abortion drugs end up in the emergency room.”
This, Hawley claimed, likely resulted in an estimated tens of thousands of women, “roughly one in 25 women” AHM claimed before the High Court as well as in their brief.
In 2023 alone, this would be potentially between 19,000 (18,638) and 30,000 (29,564) women.
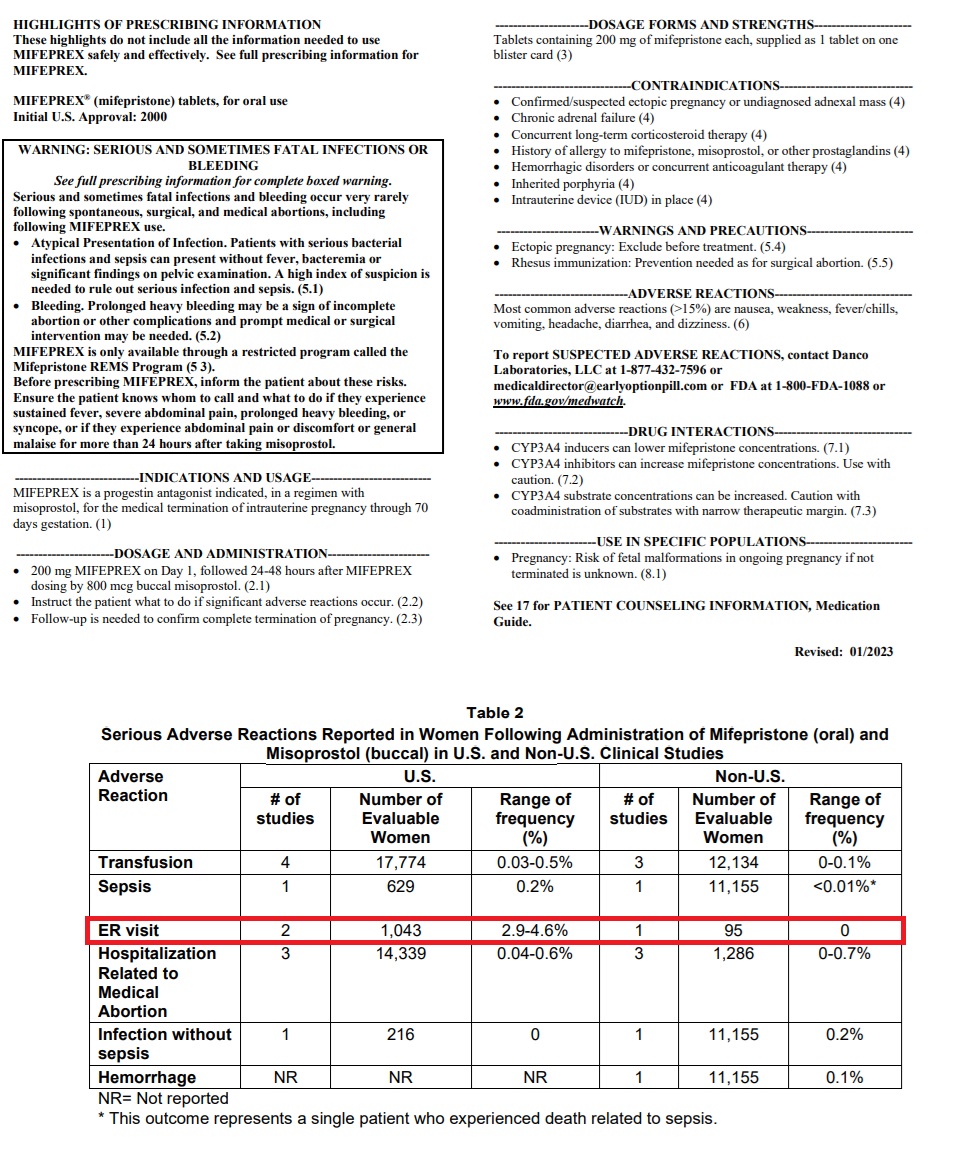
Mifepristone Jan 2023 label shows percentage of women taking abortion pill visit ER
SHOCK #3: Abortion pill manufacturer says restricting sales will ‘injure’ their business
Justice Clarence Thomas grilled Danco attorney Jessica Ellsworth on why the FDA did not take into account the Comstock Act, a federal law which prohibits the mailing of abortion-inducing drugs.
“How do you respond to an argument that mailing your product and advertising it would violate the Comstock Act?” Justice Thomas asked the abortion pill maker’s attorney.
Ellsworth dodged the question, warning Justice Thomas that challenging the FDA could “invit[e] mischief” and that the Court should “think hard” about that.
“My problem is, you’re private,” Thomas stated, claiming that private entities are not subject to a “safe harbor” against federal laws, he alleged.
Justice Alito then grilled Ellsworth, pressing into the profit motive, asking whether mifepristone was the “only product” that Danco is “currently marketing.” Alito also reiterated that the lower court’s ruling would not prohibit Danco from selling mifepristone.
“So, I gather your injury is that you think you are going to sell more if the restrictions that previously were in place were lifted,” Alito stated.
“Yes,” Ellsworth said in response.
“So, you’re going to make more money?” Alito asked.
LISTEN: Justice Samuel Alito questions whether the abortion industry is putting money over the health of women.
The Supreme Court heard a major case regarding access to the abortion pill mifepristone on Tuesday. pic.twitter.com/mVXnYcvgh8
— The First (@TheFirstonTV) March 26, 2024
“The injury is that we are prevented from selling our product in line with FDA’s scientific judgement about the safe and efficacious use of the drug,” the Danco attorney claimed.
“And you’re going to be harmed because you’re going to sell more,” Alito reiterated.
“Certainly, a company’s ability to market its product is a part of how it considers the regulatory scheme that governs its conduct,” Ellsworth said.

Danco attorney Jessica Ellsworth argues abortion pill case FDA v AHM March 26, 2024
SHOCK #4: Attorney claims non-death injury info is still collected… with zero evidence
“Do you think the FDA is infallible?” Alito asked Danco’s attorney.
“No, your honor,” Ellsworth said in response.
“Has the FDA ever approved a drug and then pulled it after experience showed that had a lot of really serious adverse consequences?” asked Alito of Danco’s counsel.
“It has certainly done that,” she responded, pointing to the adverse events reporting under the REMS.
But Alito wasn’t taking that argument. He then asked,”But don’t you think the FDA should have continued to require reporting of non-fatal consequences?”
In 2016, the FDA removed the requirement that all adverse events be reported, only requiring reporting of related deaths.
In response, Ellsworth claimed that despite the FDA’s change in 2016, Danco still gathered that information. But her claim was not backed up by any proof. “That data is part of what we are looking for all the time,” Ellsworth claimed, citing 1-800 numbers on their website and labeling.
Still, there are no requirements that anyone report abortion pill complications.
And as Live Action News has documented in our Bad Actors series, the abortion pill manufacturer has failed to decertify any prescribers who flout the REMS safety requirements, or those who suggest that women lie to emergency room staff, or those who advise ER doctors to falsify documents.
Our lawyers and clients coming out of the Supreme Court after oral arguments🙌 pic.twitter.com/bjMSDF7a3j
— Alliance Defending Freedom (@ADFLegal) March 26, 2024
Ellsworth then trotted out “tort litigation” or “deceptive advertising suits” as another possible remedy.
“Does your company think that what the FDA has done preempts state laws that prohibit the dispensation of mifepristone within their borders?” Alito asked.
“We have not taken a position on that issue,” Ellsworth replied.
“So, you don’t want to answer that question,” Alito said.







