An abortion handbook for “clinical practice” published by the World Health Organization (WHO) is promoting use of the cancer drug letrozole in combination with misoprostol to end the lives of preborn children. Yet, a U.S. clinical trial testing letrozole within the U.S. showed it to be ineffective as a chemical abortion drug.
Letrozole is the active ingredient and generic of the drug FEMARA, approved in 1997, and according to the latest label (2018), FEMARA is contraindicated for pregnancy. Drugs.com warns, “Femara can harm an unborn baby or cause birth defects. Do not use if you are pregnant.” Femara or letrozole is currently being used to treat breast cancer, and according to Drugs.com, it “lowers estrogen levels in postmenopausal women, which may slow the growth of certain types of breast tumors that need estrogen to grow in the body.”
The abortion pill mifepristone (200 mg), often referred to by the brand name Mifeprex, has only been approved by the Food and Drug Administration (FDA) for the termination of pregnancy in combination with the drug misoprostol.
In 2011, the FDA placed the abortion pill under a safety system known as REMS, which has specific requirements that the abortion lobby has tried to skirt so they can expand use of the drug. But despite the weakening of the REMS under pro-abortion presidential administrations, the abortion drug remains under this more restrictive safety program. In response, the abortion industry has tested additional drug protocols — such as the use of misoprostol alone or combining misoprostol with other drugs that are not under the REMS safety system.
World Health Organization’s Abortion Handbook
WHO’s “Clinical practice handbook for quality abortion care” is “intended to facilitate the practical application of the clinical recommendations from the Abortion care guideline (World Health Organization [WHO] 2022),” the WHO wrote.
The 2023 clinical handbook claimed, “Medical abortion is a multistep process involving two medicines (mifepristone or letrozole plus misoprostol) and/or multiple doses of one medicine (misoprostol).”
“Letrozole in a combination regimen with misoprostol is currently suggested as an alternative regimen only for abortion before 12 weeks of gestation (and not for self-management),” the handbook also reads. The World Health Organization’s medical management of abortion chart also includes the lifesaving cancer drug for use in ending the lives of preborn babies by abortion at 10 mg dosages over three days.
“Further evidence is needed to determine the safety, effectiveness and acceptability of the letrozole plus misoprostol combination regimen at advanced gestational ages, especially in comparison with that of the mifepristone plus misoprostol combination regimen (the available evidence focused on comparison with the use of misoprostol alone),” the WHO’s 2023 clinical practice handbook warned.

WHO’s abortion handbook promotes use of cancer drug
While the abortion lobby and its pro-abortion media partners are busy promoting unapproved chemical abortion protocols as well as the proliferation of illegal abortion pill syndicates shipping or walking potentially dangerous abortion drugs across the U.S. border, the WHO handbook also warned, “Anyone undergoing medical abortion must be able to access advice and emergency care in the event of complications.”
“Inform the individual that it is important for medical abortifacients to be of high pharmaceutical quality, but also that medical abortion has an inherent failure rate, even when the medicines used are of good quality and are administered correctly,” the World Health Organization abortion guidebook also stated.
Letrozole Clinical Trials In United States
Live Action News has previously documented the effort to test letrozole as well as a generic version of Lipitor as a replacement for Mifeprex in the abortion pill regimen. Another trial, which has not yet begun to recruit, plans to test use of letr0zole as a “pre-treatment” for “medical termination of pregnancy.”
The clinical trial for use of letrozole to replace Mifeprex took place at Planned Parenthood Salt Lake Health Center in Salt Lake City and was sponsored by Gynuity Health Projects (GHP). The study recruited 40 participants seeking abortions up to 63 days gestation who were given a single 30 mg dosage of letrozole (instead of World Health Organization’s recommended 10 mg over three days) followed by 800 mcg misoprostol. The trial began in January of 2022 but was completed by March of 2022.
“We applied for a grant to examine the potential of 3 new abortifacients,” principal investigator Tara Shochet, a director at GHP, told Bob Kronemyer in “Contemporary OB/GYN.”
“After exploring the published literature, we selected letrozole as the most promising. We developed the pilot study protocol based on past findings and interest in simplifying the regimen,” Shochet said.
But writing in “Contemporary OB/GYN,” Kronemyer determined that “For medication abortion, a single dose of letrozole 30 mg prior to misoprostol 800 mcg buccally is not effective, according to a pilot study published in the journal Contraception.”
That study entitled ‘Single dose letrozole and misoprostol for termination of pregnancy through 63 days’ gestation: A pilot study,‘ and funded by the OPTions Initiative, confirmed authors were seeking alternative abortion inducing drugs to utilize.
The authors wrote, “Identifying another medication that could improve upon the efficacy of misoprostol alone would benefit both abortion seekers and health systems globally. Letrozole, a nonsteroidal aromatase inhibitor (i.e., member of a class of compounds that inhibit the production of estrogen) has been identified as one such drug: it is commercially available in many countries and has been judged safe and effective for use in treating breast cancer and infertility,” the study authors wrote.
“Letrozole’s low price point ($1.80 per patient in our study versus $45 for one dose of mifepristone; written communication with site staff) and wide availability related to breast cancer treatment and infertility make it an attractive option,” the authors added. “Additionally, mifepristone is under a U.S. Food and Drug Administration Risk Evaluation and Mitigation Strategy (REMS) which imposes strict rules pertaining to its use while letrozole has no such restriction.”
“Forty people enrolled in the study between January 10 and March 8, 2022… Thirty-seven participants (93%) returned for follow-up at the study site. An additional two participants (5%) visited a non-study medical location (one visited another PPAU clinic, and one went to a local hospital emergency department) where clinicians documented clinical outcomes and shared the results with study staff,” the authors of the study also wrote.
Bob Kronemyer noted in “Contemporary OB/GYN” that the results were not very effective, writing, “Overall, 74% of patients (95% confidence interval [CI]: 60% to 89%) achieved a complete abortion; 10% ( 95% CI: 0.3% to 20%) had an incomplete abortion and opted for aspiration; and 15% (95% CI: 4% to 27%) had an ongoing pregnancy,” he wrote.
“Nineteen participants (51%) reported side effects after letrozole prior to misoprostol and two people (5%) rated these effects as severe. Side effects following misoprostol occurred in 33 participants (89%) and were as expected based on previous literature. No serious adverse events were reported,” the study authors claimed in “Contraception.”
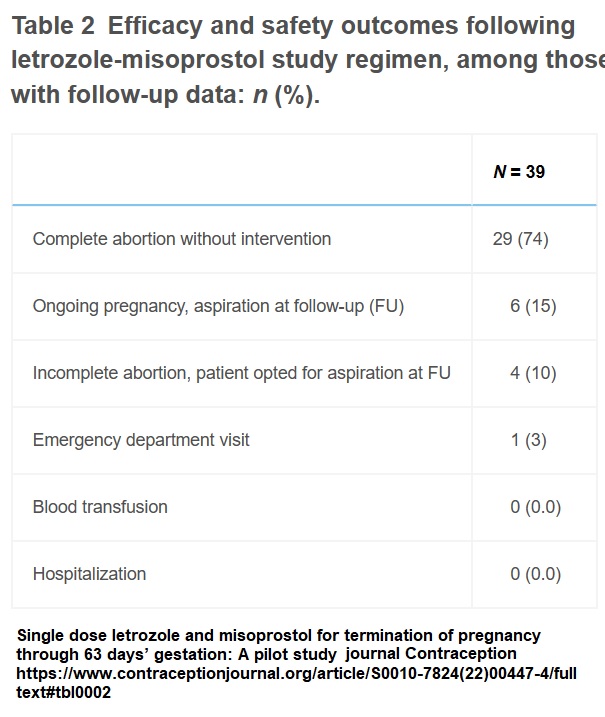
Abortion pill study used Letrozole in place of Mifeprex (Graph: journal Contraception)
“While we did not know how well the selected regimen would work, we were both surprised and disappointed by the rate of pregnancies that were not ended by the regimen,” Shochet told “Contemporary OB/GYN.”
“Although patients deemed the single dose of multiple pills acceptable, the regimen that we used was not successful enough to warrant further study,” Shochet said.








